UCD Research - April 2023
Buccal-PEP: A patch for buccal (cheek) administration of peptides
Oral administration of therapeutic peptides (large molecules) such as insulin is one of the great challenges in pharmaceutical research for human and veterinary diseases. Only five peptide-analogues have been converted to oral dosage forms such as tablets/capsules, and they offer extremely low bioavailability due to proteolytic degradation in the gut and low intestinal wall permeability. The Buccal-PEP consortium constitutes seven partners across the EU (Figure 1) and is led by Professor David Brayden at the UCD School of Veterinary Medicine
In association with


Sahil Malhotra
Post-Doctoral Fellow

Sandeep Karki
Ph.D. candidate

David Brayden
Full Professor of Advanced Drug Delivery
School of Veterinary Medicine, UCD, Belfield, D04 V1W8; Coordinator of BUCCAL-PEP
The consortium has been awarded €3.7 million to develop a multifunctional biomaterial patch which allows buccal (in the cheek) delivery of peptide-analogue therapeutics. This will overcome some of the drawbacks of oral administration, specifically to reduce interaction with food and to avoid peptide breakdown in the gut and liver. The team will integrate permeation enhancers and a Glucagon-Peptide-1(GLP-1) analogue in a multilayered buccal patch to treat Type II diabetes, a major disease for humans and small animals. The aim of Buccal-PEP is to develop next generation buccal patches to deliver the peptide-analogues to the blood via the buccal cavity.
The proposed structures will be biocompatible and will be engineered to release the payload in a controlled fashion using selected biomaterials. The buccal patch will be designed to significantly enhance the bioavailability of peptide drugs in comparison to their injected counterparts while ensuring patient adherence to dosing requirements. Mechanistically, the patch will enable peptide analogues to diffuse across the mucosal multilayer, thereby effectively achieving the intended pharmacological response.
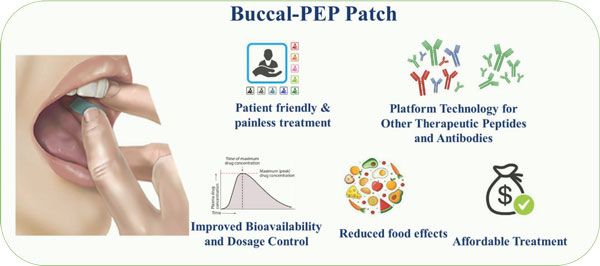
The Buccal-PEP patch.
Buccal route for peptide delivery
Use of the buccal (inner cheek) mucosa for administering peptides has some advantages. It is relatively permeable compared to other sites in the oral cavity; it is highly vascularised, and this allows rapid absorption of the drug into the systemic circulation and circumvents hurdles of oral administration including the stomach environment and liver first-pass metabolism1. But owing to the structure of the buccal epithelium, it still possesses a high permeability barrier for the complex and high molecular weight peptides to diffuse across the tissue and enter the submucosa (Figure 2). The buccal epithelium is stratified and is ~40-50 cell layers thick, with tightly packed intercellular spaces, mostly enriched with a distinct combination of lipids and structural proteins. Thus, it possesses a great permeability barrier for the free diffusion of hydrophilic and high molecular weight macromolecular drugs such as peptides2.
The uniqueness of this project lies in the careful selection of biocompatible permeation enhancer Sodium Glycodeoxycholate (SDC), which the researchers have previously shown to enhance the fluxes of hydrophilic macromolecule FD4, by modulating the permeability of buccal epithelium. The project aims to meticulously design the buccal patch, co-loaded with the peptide drug, permeation enhancers and other excipients, that will temporarily adhere to the cheek and enable the uptake of drug molecules across buccal mucosa. The patch will be developed with Generally Regarded as Safe (GRAS) polymers such as cellulose derivatives, and will disintegrate in just 15 to 30 minutes. The patch will be designed to decrease Foreign Body Sensation and thus increase the acceptability by human patients in the first instance, while maintaining the required blood levels linked to the therapeutic response.

Figure 1: The seven BUCCAL-PEP consortium partners include academic groups working on patch design, a big Pharma to provide peptides, a patch maker, experts in pig studies, and regulatory experts from across Europe.
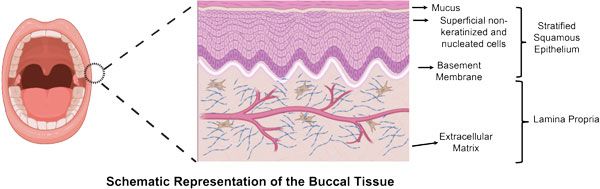
Figure 2: Graphical representation of human buccal (cheek) tissue. Pig cheeks provide the closest animal model representation of human tissue. The patch will be applied to the surface of the buccal mucosa inside the mouth.
Design of the buccal patch
Buccal-PEP is proposed to be a triple-layered patch that adheres to the buccal epithelium. The patch contains the peptide, a nominated permeation enhancer, and excipients to stabilise the GLP-1. In a dry environment, there is no flow of molecules through and from the patch. However, a moist environment will cause the layers to swell, initiating a flow of molecules through the layers of the patch and into the epithelium. The patch encompasses three layers (Figure 3):
1. A mucoadhesive layer
This allows the patch to stick to the inner cheek. This layer will swell due to contact with the moist environment, allowing for the permeation enhancer (PE) to be released. It is based on a mix of the biodegradable polymers, chitosan, and cellulose derivatives.
2. A middle layer
This contains the GLP-1 analogue and a peptidase inhibitor to protect against salivary enzymes. The flow of molecules through this layer will initiate once the mucoadhesive layer has sufficiently swollen, inducing a short time delay in release of compounds between the two layers.
3. A backing layer
This ensures unidirectional flux by wrapping around the other two layers. It is made of methyl/ethyl cellulose and will disintegrate in the saliva more slowly than the other layers. To further ensure unidirectional flux in the direction towards the buccal epithelium, the backing layer will be extended laterally around the two other layers, thereby preventing lateral flux.
The consortium will design a lead GLP-1 product (with Type 2 diabetes as the showcase indication) that will be validated for evidence of sufficient blood levels and lack of damage to the buccal tissue in pig studies. Additionally, a Health Technology Assessment will be performed to support an evidence-based, value proposition and aid in the development of a commercialisation strategy.
The final deliverable of the project is a patch that is pre-clinically validated and ready for human clinical trials. Overall, this project will provide a platform technology for oromucosal delivery of peptide-analogues that will be suitable for a broad range of peptide analogue therapies across a multitude of disease indications. If successful, there may be opportunities for subsequent oral cavity device designs for feline and canine patients, thus adhering to principles of One Health.
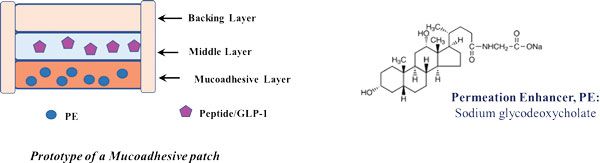
Figure 3: Buccal patch prototype and its design.
References
- Morales JO, Brayden DJ. Buccal delivery of small molecules and biologics: of mucoadhesive polymers, films, and nanoparticles. Curr. Opin. Pharmacol. 2017;36:22-28. doi: 10.1016/j.coph.2017.07.011.
- Varsha V. Nair, Pablo Cabrera, Constanza Ramírez-Lecaros, Miguel O. Jara, et al. Buccal delivery of small molecules and biologics: of mucoadhesive polymers, films, and nanoparticles – an update. Int. J. Pharm. 2023:122789. doi: 10.1016/j.ijpharm.2023.122789.

Funding statement
This project has received funding from the European Union’s Horizon Europe research and innovation programme under grant number 101071054.
In association with


Sahil Malhotra
Post-Doctoral Fellow

Sandeep Karki
Ph.D. candidate

David Brayden
Full Professor of Advanced Drug Delivery
School of Veterinary Medicine, UCD, Belfield, D04 V1W8; Coordinator of BUCCAL-PEP















