Cerebral Ischaemic Infarction in a dog diagnosed by MRI
Rosie Ellis DVM GPCert(DI) MSc BA, founder of Ultrasound Vet, reports on a case study she worked on as a diagnostic imaging intern
A twelve-year-old male neutered Shih Tzu was urgently sent to our referral service subsequent to presentation at his primary care practice (PCP) for an acute onset of seizure episodes and a markedly abnormal neurological examination. Complete blood count (CBC) and serum biochemistry were within normal limits. Once the patient was stabilised, an MRI was performed which established a right hemispheric cerebral ischaemic infarct. Unremarkable results from cerebrospinal fluid (CSF) analysis, additional blood work (C-reactive protein (CRP), total T4), urine sampling (urinalysis, urine protein creatinine ratio (UPC)) and repeated blood pressure measurements suggested that the patient had probably suffered an idiopathic ischaemic infarction of the right cerebral hemisphere. Following anti-seizure treatment and adjunctive therapies, the patient continued to make a full recovery over four weeks.
Introduction
Cerebrovascular accidents (CVAs) are increasingly diagnosed as a cause of acute, non-progressive, focal central nervous system dysfunction in dogs (Thomas et al, 1996; Hillock et al 2006; Arnold et al, 2020). Wessmann et al, (2009), reported that 1.5 – 2 per cent of neurological referral cases were diagnosed with a CVA. They suggest that an increase in documentation of CVAs will arise as advanced imaging modalities become more commonplace in veterinary practice (Choi et al, 2018).
Commonly referred to as strokes, CVAs can be haemorrhagic or ischaemic. The majority of CVAs in dogs are ischaemic in nature (Cervera et al, 2010; Boudreau, 2018) and, subsequently, focal tissue infarction can occur (Arnold et al, 2020). Inadequate blood flow to a region of the brain causes a portrait of clinical signs that are determined by the topographic location and size of the infarct (Thomsen et al, 2017). In dogs, infarction is most commonly associated with the rostral cerebellar artery (Arnold et al, 2020), with other arteries of the Circle of Willis or the caudal cerebellar artery being affected to a lesser extent (Garosi et al, 2006).
There are a variety of diseases in dogs that are associated with the development of a CVA. Endocarditis, sepsis, primary or metastatic neoplasia and disorders that are associated with systemic hypertension or hypercoagulability are among the more common (Arnold et al, 2020; Garosi, 2010). A study by Garosi et al (2005), demonstrated that 54.5 per cent of patients with a confirmed CVA had a co-morbidity, additionally stating that either chronic renal disease or undiagnosed hyperadrenocorticism were the most frequently encountered concomitant pathologies. However, no correlation has been proven between the type and location of an infarct and a specific co-morbidity (Garosi et al, 2005).
Diagnosis of a CVA requires cross-sectional neuroimaging. MRI is considered a highly sensitive and specific imaging modality for the detection and differentiation of infarcts. Although computed tomography (CT) is an appropriate technique to highlight acute haemorrhage, MRI is the modality of choice for diagnosing ischaemic infarction (Platt & Garosi, 2003; Boudreau, 2018).
Utilising MRI, ischaemic infarcts have been typically considered to be homogenous well-demarcated wedge-shaped to round lesions. They are characteristically hypointense to surrounding parenchyma in T1-weighted images and hyperintense on T2-weighted images and fluid attenuated inversion recovery (FLAIR) images (Cervera et al, 2010; Arnold et al, 2020). Contrast enhancement can be optimally noted in images obtained seven to 10 days following a CVA, however this may be visualised as early as 24 to 48 hours following the onset of neurological signs (Cervera et al, 2010; Boudreau, 2018).
History
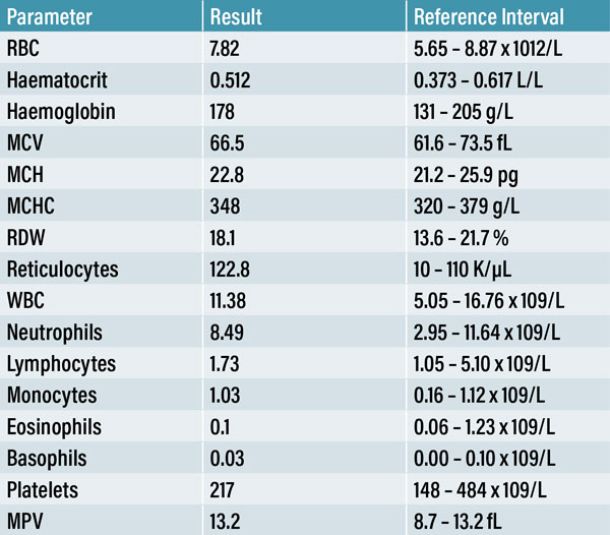
A twelve-year-old male neutered Shih Tzu dog was referred to our emergency service following an acute presentation of cluster seizures at his PCP. An in-house CBC (Table 1), serum biochemistry and electrolyte panel (Table 2) were obtained. Apart from a mild reticulocytosis of 122.8 K/µL (reference range: 10-110 K/µL), no other abnormalities were noted. The PCP administered rectal diazepam and continued with an oral prescription of an anti-seizure medication. As the seizure activity continued throughout the day, further diagnostics were advised. The patient was sent to our emergency referral service within eight hours of the onset of seizures.
There was no known access to toxins and no trauma that the owner was aware of. There were no previous seizures or neurological deficits in the history and no current clinical conditions requiring medication. Dermatological disease had previously been treated with Oclacitinib Maleate (Apoquel film coated tablets, Zoetis UK Limited) with the last prescription concluded five months prior.
The owner reported that the previous day the patient had been clingy, was seeking out corners and exhibiting head-pressing behaviour. Cluster seizures presented as repeated generalised tonic-clonic contractions, paddling of all four limbs, unconsciousness and autonomic signs such as incontinence and salivation. The owner noted that the patient did not appear to be aware of his surroundings during these episodes. The patient was consistently circling to the right while demonstrating contralateral marked fore-limb, and possibly mild hind-limb, paresis. The owner was concerned that he was blind since the onset of seizures.
Clinical Findings
The dog was quiet and reasonably responsive on presentation. On physical examination, the mucosa was pink with a normal capillary refill time (< two seconds). The heart rate was 100 beats per minute and a grade I/VI left-sided systolic heart murmur was noted on auscultation. Uniform femoral pulses were palpated and correlated to the heart rate and rhythm. Although the dog was panting, thoracic auscultation and respiratory effort were normal. Abdominal palpation was comfortable and the body condition normal. The patient was normothermic (rectal temperature: 38.1oC). No palpable lymphadenopathy was appreciated.
The patient had a right-sided head tilt and circled to the right continuously. Palpebral and pupillary light reflexes were present bilaterally and the menace reflex was bilaterally decreased. No nystagmus was noted. The dog had noticeable left forelimb paresis with a marked decrease in conscious proprioception (> three seconds). Spinal reflexes were intact. No pain was elicited on palpation of the vertebral column and there was normal range of motion of the neck.
Problem list and differential diagnoses
Problem list:
- Acute onset of cluster seizures
- Circling
- Head tilt
- Paresis contralateral to head tilt
Differential diagnoses for seizures and neurological signs associated with forebrain dysfunction:
Intracranial:
- Structural:
- Space occupying lesion (neoplasia, cyst, abscess)
- Inflammation (encephalitis, meningitis)
- Infection (bacterial, viral, protozoal)
- Metabolic storage disease
- Vascular event
- Scar tissue
- Trauma (hematoma, contusion)
- Congenital disorder (chiari malformation, hydrocephalus).
- Functional:
- Idiopathic epilepsy.
Extracranial:
- Metabolic disorder
- Hepatic / Renal encephalopathy
- Electrolytes imbalances
- Hyperviscosity syndrome
- Toxicity (lead, metaldehyde, organophosphates, ethylene glycol, serotonin).
Diagnostic Techniques
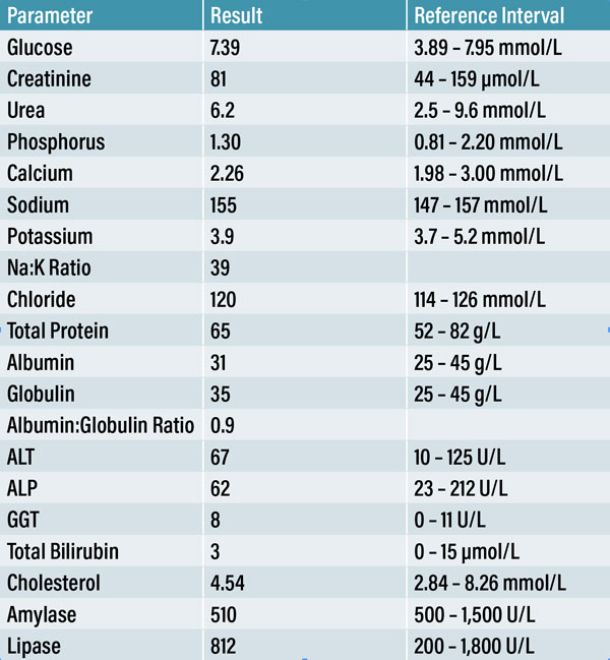
A CBC (Table 1), serum biochemistry and electrolyte panel (Table 2) were performed at the PCP to evaluate extracranial causes of seizures. As these findings were within normal limits, an intracranial pathology, more specifically a lesion of the forebrain, was considered to be the most likely cause for this patient’s presentation. Following a comprehensive neurological examination, it was decided to perform a contrast MRI brain study. Although CT was also available on-site, MRI delivers more detailed information on intracranial structures (Platt & Garosi, 2003).
A 22G intravenous catheter was placed in the right cephalic vein. Once clinically stable, the patient was pre-medicated with 0.2mg/kg midazolam (5mg/ml solution for injection, Hameln Pharmaceuticals) and 0.2mg/kg butorphanol (Torbugesic 10mg/ml solution for injection, Zoetis UK Limited) intravenously (IV) and induced with
1-4mg/kg propofol (PropoFlo Plus 10mg/ml emulsion for injection, Zoetis UK Limited) given IV to effect. The patient was intubated (6.5mm endotracheal tube), positioned in sternal recumbency and placed on a Lack breathing circuit maintained on 0.5-1.5 per cent Isoflurane (Iso-Vet 1000mg/g inhalation vapour liquid, Chanelle Pharma) with oxygen at 2L/minute. Multi-parameter monitoring (Mindray iPM12 Vet Monitor) included capnography, blood pressure, pulse oximetry and electrocardiogram, the last of which was removed during the MRI. Intravenous fluid therapy was commenced (Hartmann’s Lactated Ringers solution for infusion, B. Braun Vet Care) at twice maintenance (4ml/kg/hour).
Additional venous blood samples were retrieved and sent to TDDS Laboratory for assessment of CRP and total T4. A cystocentesis was performed with ultrasound guidance (Esaote MyLab 30 and Esaote CA123 micro-convex probe) for a complete urinalysis while the patient was under general anaesthesia.
The MRI study utilised a 0.2T MRI machine (Esaote Opera E-Scan XQ) located on the premises. The patient’s head was placed within a circular extremity coil. Half Fouriers (HF) were obtained in three planes (dorsal, sagittal and transverse) to assure adequate positioning of the patient prior to scanning. A brain sequence was implemented under the guidance of a board-certified radiologist. A matrix of 256 x 256, slice thickness of 4mm and gap of 0.4mm were selected for all sequences. Gadopentetate Dimeglumine (Magnevist 0.5mmol/mL solution for injection, Bayer Healthcare), a paramagnetic contrast enhancement agent, was given intravenously at a dose of 0.5ml/kg.
Brain MRI sequence protocol:
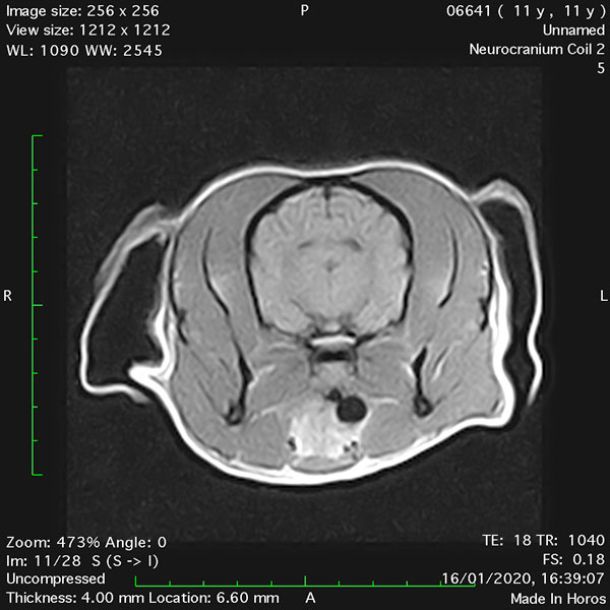
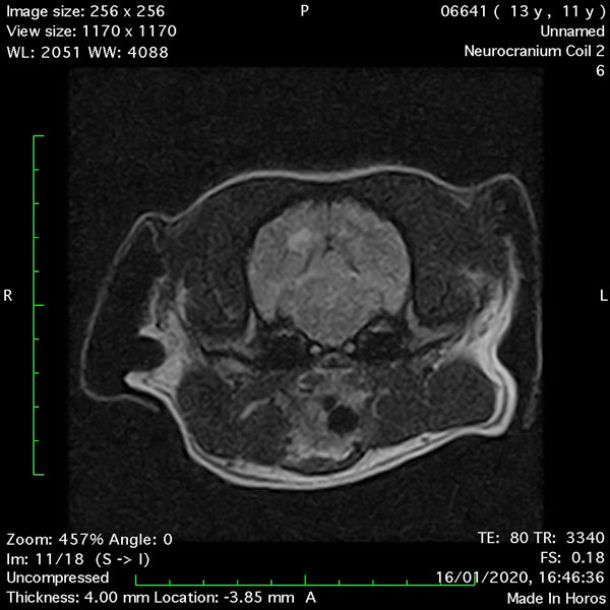
- Transverse T1 – Spin Echo T1 (Figure 1);
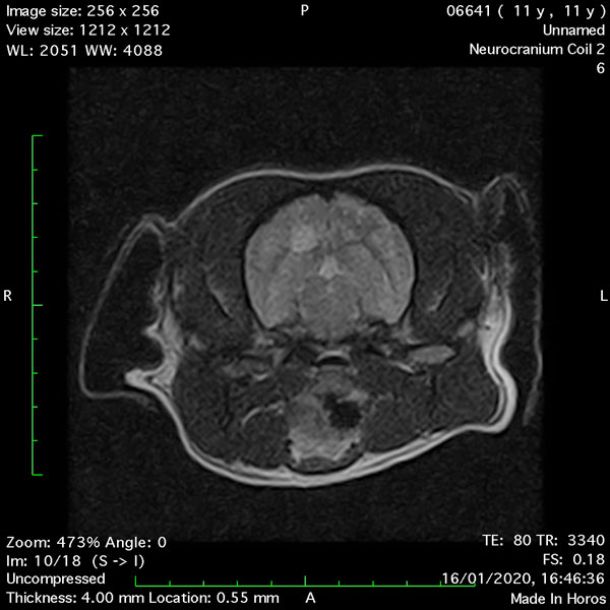
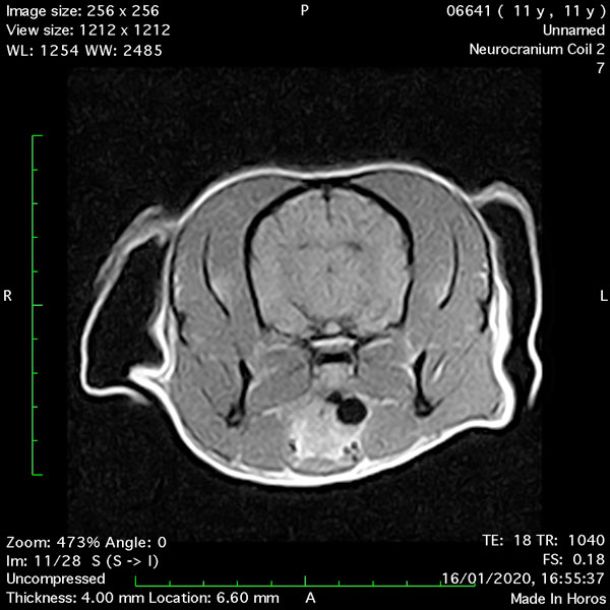
- Transverse T2 – Turbo Spin Echo-S (contrast administered slowly during this sequence) (Figure 2) (Figure 3);
- Transverse T1 – Spin Echo T1 (post-contrast) (Figure 4);
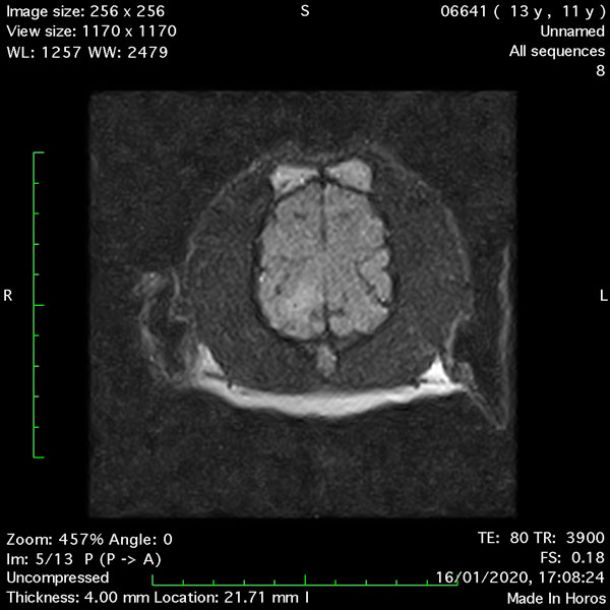
- Dorsal FLAIR (fluid-attenuated inversion recovery) (Figure 5);
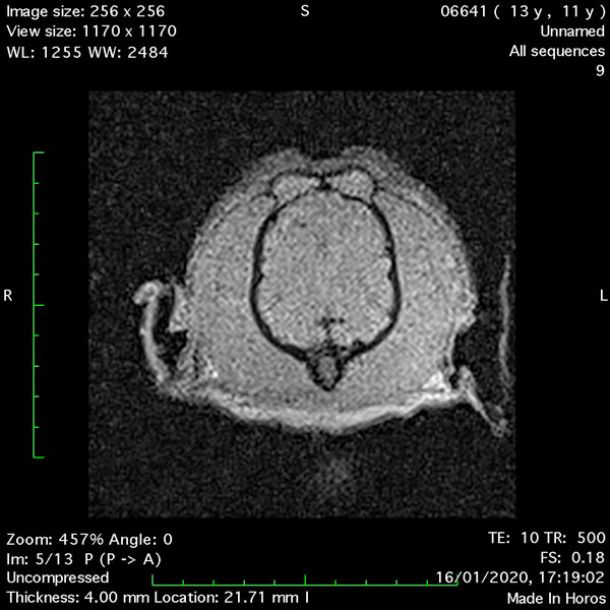
- Dorsal Gradient Echo 10ms (Figure 6);
- Sagittal T2 – Turbo Spin Echo-S (Figure 7);
MRI images were viewed in DICOM format (Digital Imaging and Communications in Medicine) (Figures 1-7) and assessed in-house under the guidance of a board-certified radiologist. The series were evaluated for changes in intensity in the T1- and T2-weighted sequences (T1w and T2w, respectively). There was a 4mm x 7mm x 7mm intra-axial, wedge-shaped hyperintense reasonably-well demarcated region in the right cerebrum visible in the T2w images (Figures 2-3). This was consistent with a focal infarct. No contrast enhancement, anatomical abnormality nor space occupying lesion was noted in the T1w sequences (Figure 1, Figure 4). There was no observed pathology on assessment of the FLAIR images (Figure 5).
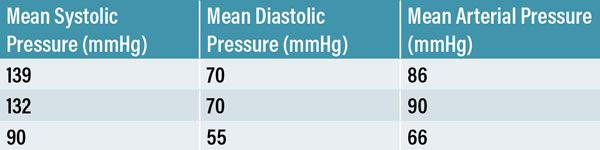
Table 3: Non-invasive blood pressure measurements (Mindray iPM12 Vet Monitor) were obtained using a size 3 cuff placed on the right proximal antebrachium. Mean blood pressure measurements summated from six readings were taken at three separate occasions while in hospital.
A CSF tap at the cerebellomedullary cistern was performed by a board-certified surgeon while the patient was under general anaesthesia and the sample sent to TDDS Laboratory. The CSF analysis reported no inflammatory cells, infectious agents or overt evidence of neoplastic disease, although it was noted that this cannot definitively exclude intracranial neoplasia.
The additional blood samples were reported the following day. CRP returned as mildly elevated (24mg/L) (reference range: 0-10mg/L) and therefore was not indicative of an active meningitis or encephalitis. A total T4 returned within normal limits (18nmol/L) (reference range: 15-50nmol/L).
Urinalysis indicated a mild presence of erythrocytes and epithelial cells, which can result from the sampling technique. There was evidence of struvite crystalluria, considered an incidental finding. The remaining parameters were within normal limits and the UPC ratio was 0.13 (reference range: 0.0 – 1.0).
Non-invasive blood pressure measurements (Mindray iPM12 Vet Monitor) were obtained at three separate occasions during hospitalisation. A size 3 cuff was placed on the right antebrachium. Mean values were calculated from six consecutive readings taken at three separate occasions while hospitalised (Table 3).
Diagnosis
There are a multitude of potential causes for an acute neurological presentation with evidence of forebrain dysfunction. The absence of any significant changes on bloodwork, urinalysis and CSF analysis, in conjunction with the MRI findings, led us to the diagnosis of an idiopathic ischaemic CVA.
Treatment
As there is no specific therapy available that significantly affects the outcome of a CVA in dogs (Platt & Garosi, 2003; Garosi, 2010; Arnold et al 2020), the treatment plan focused on management of neurological episodes, supportive care and maintenance of adequate perfusion.
A total loading dose of 18mg/kg Phenobarbital Sodium (200mg/ml injection, Martindale Pharma) was initially delivered slowly IV at a preliminary dose of 12mg/kg and a subsequent dose of 6mg/kg until the seizure activity ceased. Phenobarbital was then continued by mouth (PO) twice daily to control seizures. One mg/kg Maropitant (Prevomax 10mg/ml solution for injection, Dechra Veterinary Products) was administered IV once daily to decrease nausea. Intravenous fluid therapy was continued to ensure sustained perfusion.
The patient was hospitalised for three days where no further seizure activity was noted. He improved daily and regained mobility demonstrating a gradual capacity to walk straight for short periods of time and turn his head both left and right. Recheck consultations were set up for one week and two weeks’ post-hospitalisation, following which care was transferred back to the PCP.
The patient was discharged with prescriptions of 2mg/kg Maropitant (Cerenia tablets, Zoetis UK Limited) PO once daily for three days and a 9kg dose of Meloxicam (Metacam 1.5mg/ml oral suspension, Boehringer Ingelheim Animal Health UK Limited) PO once daily for two days as an anti-inflammatory.
2.5mg/kg Phenobarbital (Phenoleptil tablets, Dechra Veterinary Products) was prescribed PO twice daily for three weeks with a three-week tapering period. The owner was given a 10mg rectal diazepam (Stesolid rectal solution, Balkanpharma – Dupnitsa AD) to administer in case of seizures. Strict instructions were issued for cage rest and harness walks until the recheck.
Due to the presence of urinary struvite crystals, it was recommended to commence a prescription diet with an S/O index. A diet high in Omega-3 and essential fatty acids can be recommended in patients with cerebral infarcts (Ozen et al 2008).
Regular blood pressure monitoring was recommended and, in case of hypertension, a prescription of 0.5mg/kg Enalapril given PO twice daily was suggested.
Prognosis and outcome
CVAs usually have a peracute onset and the clinical condition is typically non-progressive after 24 hours, unless secondary changes occur due to cerebral oedema. The outcome is dependent upon lesion location and management of the resultant neurological impairments (Platt & Garosi, 2003). It is paramount to investigate any co-morbidities that may predispose the patient to an ischaemic event as these may increase the risk of successive infarcts (Boudreau, 2018).
Discussion
The characteristics of an infarct in both MRI and CT correspond to the time since infarction (Platt & Garosi, 2003). Choi et al, in 2018 determined the effect of time on the appearance of canine brain infarctions employing sequential MRI studies. Delineation and signal strength of a CVA on T2w images had a positive correlation to the time since the onset of clinical signs. Interestingly, they reported that cerebral lobe lesions were best noted on FLAIR images at least 24 hours post-occlusion. It could be surmised that had the MRI for our patient been postponed, evidence of infarction on the FLAIR image may have been more readily observed. Furthermore, Choi et al, (2018) state that diffusion-weighted imaging (DWI) can aid in early detection of ischaemic disease. This was not implemented in this MRI study.
There is no specific treatment for ischaemic infarcts and therapy focuses on supportive care and prevention of further neurological damage (Garosi et al, 2005; Arnold et al, 2020). It was important to investigate and address any underlying condition that may have predisposed the patient to a CVA and to treat as necessary. With remarkably normal blood, urine and CSF results, and a mean arterial pressure within normal limits, we concluded that our patient had probably developed an idiopathic ischaemic CVA. An ACTH stimulation test could have been performed to further exclude a peripheral hyperadrenocorticism.
Data is sparse on the long-term morbidity and mortality of dogs with CVAs, although the prognosis is considered good as over two-thirds of patients make a significant improvement or return to normal (Garosi et al, 2005).
The diligent nature of the owners fortunately allowed for a comprehensive diagnostic work up and the patient continues to be clinically well.
Note
Figures 1-7: Images obtained with MRI depicted in sequential order and assessed under the guidance of a board-certified radiologist in-house.
Arnold, S., Platt, S., Gendron, K., & West, F. (2020). Imaging ischaemic and hemorrhagic disease of the brain in dogs. Front Vet Sci 7 (279), pp. 1-18.
Boudreau, C. (2018). An update on cerebrovascular disease in dogs and cats. Vet Clin North Am Small Anim 48 (1), pp. 45-62.
Cervera, V., Wilfried, M., Charles, V., Johnson, V., Dayrell-Hart, B. & Seiler, G. (2010). Comparative magnetic resonance imaging findings between gliomas and presumed cerebrovascular accidents in dogs. Veterinary Radiology & Ultrasound 52 (1), pp. 33-40.
Choi, S., Noh, D., Kim, Y., Jeong, I., Choi, H., Lee, Y. & Lee, K. (2018). Magnetic resonance imaging characteristics of ischaemic brain infarction over time in a canine stroke model. J Vet Sci 19 (1), pp. 137-142.
Hillock, S., Dewey, C., Stefanacci, J. & Fondacaro, J. (2006). An in-depth look: vascular encephalopathies in dogs: incidence, risk factors, pathophysiology and clinical signs. Neurology Compendium 28 (3), pp. 1-14.
Garosi, L., McConnell, J., Platt, S., Barone, G., Baron, J., de Lahunta, A. & Schatzberg, S. (2005). Results of diagnostic investigations and long-term outcome of 33 dogs with brain infarction (2000-2004). J Vet Intern Med 19 (5), pp. 725-731.
Garosi, L., McConnell, J., Platt, S., Barone, G., de Lahunta, A. & Schatzberg, S. (2006). Clinical and topographic magnetic resonance characteristics of suspected brain infarction in 40 dogs. J Vet Intern Med 20 (2), pp: 311-321
Garosi L. (2010). Cerebrovascular disease in dogs and cats. Vet Clin North Am Small Anim Pract 40 (1), pp. 65-79.
Ozen, O., Cosar, M., Sahin, O., Fidan, H., Eser, O., Mollaoglu, H., Alkoc, O., Yaman, M. & Songur, A. (2008). The protective effect of fish n-3 fatty acis on cerebral ischaemia in rat prefontal cortex. Neurol Sci 29 (3), pp. 147-152.
Platt, S. & Garosi, L. (2003). Canine cerebrovascular disease: do dogs have strokes? J Am Anim Hosp Assoc 39 (4), pp. 337-342.
Thomas, W., Sorjonen, D., Scheuler, R. & Kornegay, J. (1996). Magnetic resonance imaging of brain infarction in seven dogs. Veterinary Radiology & Ultrasound 37 (5), pp. 345-350.
Thomsen, B., Gredal, H., Wirenfeldt, M., Kristensen, B., Clausen, B., Larsen, A., Finsen, M., Berendt, M. & Lambertsen, K. (2017). Spontaneous ischaemic stroke lesions in a dog brain: neuropathological characterisation and comparison to human ischaemic stroke. Acta Vet Scand 59 (7), pp. 1-9.
Wessmann, A., Chandler, K. & Garosi, L. (2009). Ischaemic and hemorrhagic stroke in the dog. Vet J 180 (3), pp. 290-303.
1. Select the false answer with regard to cerebrovascular accidents:
A. They can be of a haemorrhagic or ischaemic nature
B. Clinical signs depend upon the location and size of the infarct
C. CVAs are typically haemorrhagic in dogs
D. The rostral cerebellar artery is the most commonly affected vessel
2. What are the two most common disorders associated with the development of a CVA?
A. Chronic renal disease and undiagnosed Hyperadrenocorticism
B. Neoplasia and undiagnosed Hypoadrenocorticism
C. Polycythaemia and Neoplasia
D. Trauma and DIC
3. Which imaging modality is considered the most sensitive for detection of an ischaemic CVA?
A. X-ray
B. MRI
C. CT
D. Contrast Enhanced CT
4. How early may contrast enhancement of the lesion be noted on MRI following the onset of neurological signs?
A. 24-48 hours
B. 72 hours
C. One week
D. One month
5. Select the false answer regarding treatment options for ischaemic CVAs:
A. There is no specific treatment for infarcts
B. It is important to focus on supportive care of the patient
C. Treatment may include prevention of further neurological damage
D. Investigating and addressing the underlying cause of a CVA is of little importance clinically
ANSWERS: 1C; 2A; 3B; 4A; 5D.