Clinical approach to the acute neurological intoxication
Acute intoxication is a common clinical presentation in first-opinion practice with numerous toxins that can lead to neurological signs. In this article, Sarah Butterfield BSc(Hons) BVSc PGDip(VCP) MRCVS, Senior Clinical Training Scholar in Neurology and Neurosurgery, Queen Mother Hospital for Animals (RVC London) aims to provide a basic overview of the clinical presentation of a patient with neurotoxicity, and to highlight the most common neurotoxins, the general approach to diagnostic testing and treatment strategies
Introduction
Intoxication is a common emergency presentation in first-opinion practice with many presenting with neurological signs. This may be termed primary (via direct effect) on the nervous system or secondary (indirect effect) following dysfunction of other organs, such as in hepatic or uraemic encephalopathy1.
Neurotoxins can also be classified as neuro-excitatory or neuro-inhibitory. Excitatory toxins typically cause hyperexcitability, seizures, muscle tremors, fasciculations and ataxia. Secondary complications include hyperthermia, disseminated intravascular coagulation (DIC) and rhabdomyolysis. Neuroinhibitory toxins can cause obtundation, stupor, coma and/or weakness or flaccid paralysis leading to respiratory failure1.
Diagnosis is often based on clinical history (if known exposure or acute onset of signs, e.g., after a walk), and/or clinical signs consistent with exposure and appropriate diagnostic testing. The exact toxin ingested is not always known and treatment in the emergency setting is often based on a presumptive diagnosis. Prompt recognition and appropriate emergency treatment is required to ensure a good outcome.
Clinical approach to a suspected intoxication
Taking a history
It is important to take a thorough clinical history to assess the likelihood of intoxication. Typically, the signs are acute in onset in a previously healthy animal. It may be possible to discount a toxin as a likely cause of the neurological signs if, for example, the patient presents with a progressive neurological presentation (over days to weeks) or following multiple seizure events over a prolonged period of time with normal inter-ictal period. If the toxin is known, exact ingredients, possible dosage exposed to, and timing of exposure should be ascertained on admission.
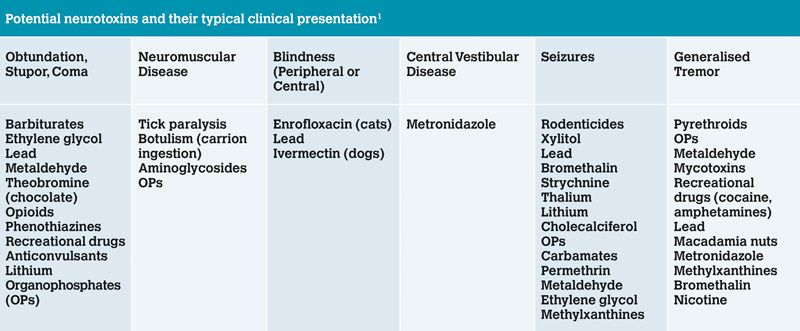
Table 1: Potential neurotoxins listed by typical clinical presentation. Note this list is not exhaustive and there are many additional toxins that may cause neurological signs in animals
Clinical Examination
Common neurological signs and neurotoxins are listed in Table 1. A full assessment of the patient should be made and any immediate life-threatening complications addressed, such as hypovolaemia, cardiac rhythm abnormalities or hypo/hypertension. Respiratory rate and effort should be assessed, and in cases of respiratory compromise, a patent airway secured and ventilation provided.
Diagnostic Testing
In the majority of cases, a definitive diagnosis is not possible and treatment is presumptive and supportive. Blood analysis should be performed including a minimum emergency database, comprehensive haematology and serum biochemistry. This may assist in identifying abnormalities that indicate a specific toxin causing systemic disruption and guide our treatment plan. Examples include acid-base disturbance such as metabolic acidosis and calcium oxalate monohydrate crystalluria in cases of ethylene glycol, severe azotaemia in uraemic encephalopathy, elevated liver parameters (including bile acid and ammonia) in hepatic encephalopathy, prolonged PT/APTT and activated clotting times in coagulopathies, and severe hypoglycaemia in cases of xylitol toxicity. It is suggested to collect plasma, serum and urine samples for freezing which may then be sent to an external laboratory for comprehensive toxicology. However, such analysis may take up to 10 days and treatment should not be delayed pending these results. If vomiting is a clinical feature or is induced as part of treatment (see below), the vomitus may also be visually inspected or material frozen for analysis.
Treatment of a suspected intoxication:
With any intoxication, a general treatment approach may be followed (Figure 2). However, indications for specific therapies should be taken into account with individual patients.
Stabilisation of vital parameters and neurological signs
The patient must be assessed and any immediate treatment administered if required. Important considerations include seizure management, severe neuromuscular signs with subsequent respiratory compromise and raised intracranial pressure. The latter, although unlikely following neuro-intoxication, may be suspected if there is reduced or progressively deteriorating mental status, miosis, anisocoria or mydriasis. It is also possible to see progressive bradycardia, hypertension and an abnormal breathing pattern, also termed Cushing’s response or triad, which is evidence of severe or even terminal brain stem dysfunction.
The Modified Glasgow Coma Scale can also be used to facilitate patient monitoring and early detection of deleterious trends. If an elevation in intracranial pressure is suspected, treatment should be initiated with either Mannitol (0.25-1g/kg of 10 per cent solution over 10-15 minutes) or 7 per cent hypertonic saline (4ml/kg in dogs, 2ml/kg in cats IV over 15 minutes) and the head elevated to encourage venous drainage. Blood sampling using the jugular vein should be avoided. Care should be taken using Mannitol in cases of hypovolaemia and dehydration due to risk of acute kidney injury, and avoidance using hypertonic saline in patients with hypernatraemia. You should always follow these with isotonic fluids to restore fluid balance following diuresis, to restore electrolytes and prevent hyperosmolarity.
Seizures are a common clinical sign of neuro-toxicity and prompt treatment is vital to avoid secondary complications, such as long-term cerebral injury or heat stroke. Figure 1 describes an approach to emergency seizure management. If the patient is actively tremoring or showing evidence of muscle fasciculations, Methocarbamol may be used, either by an IV (currently unavailable in the UK) or oral preparation (44–220mg/kg IV, given in boluses of 30–40mg/kg to a maximum of 330mg/kg/day1 or 20-45mg/kg PO every eight hours)2. If the patient is comatose or lacking a gag reflex, the oral preparation may be diluted in water and given rectally. This is particularly useful in cases of tremorgenic mycotoxins following ingestion of mouldy food.
Intravenous benzodiazepines may also be considered as anti-epileptic therapy (see Figure 1), for skeletal muscle relaxation, anxiolysis and sedative effects. Caution must be taken in cases of hepatic damage due to their metabolism by the liver. Also beware of the risk of acute hepatic fulminant necrosis in cats reported following repeated oral administration.
Prevent continued absorption of the toxin
Next, we must consider how to prevent continued absorption of the toxin, typically performed by inducing emesis if within three hours of ingestion. Contraindications to emesis include altered mentation, loss of gag reflex, seizures, coma, or ingestion of caustic substances. Should this be the case, gastric lavage may be considered as an alternative under general anaesthesia (see emergency texts for technique). It is important to stabilise the patient prior to anaesthesia and to make a risk-benefit assessment on the risks of an anaesthetic versus continued absorption of the toxin. In the case of percutaneous toxins, most notably permethrin toxicity in cats, decontamination of the coat is advised. In mild intoxications, treatment with oral activated charcoal alone may be sufficient.

Figure 1: Fundamentals of emergency seizure management including specific drugs used.
Enhance elimination of the absorbed toxin
Attempts must be made to enhance the clearance of the ingested toxin. Intravenous fluid therapy is indicated to ensure diuresis. Care must be taken in patients with concurrent cardiac disease, i.e., to avoid fluid overload, hypertension or acute renal failure. Placement of a urinary catheter could be considered, and can often be performed without sedation in males, to accurately measure urinary output. This is particularly of use following chocolate ingestion due to the continued absorption of metabolites across the bladder wall. A metoclopramide continuous rate infusion (CRI) at 1-2mg/kg/day has also been described to increase gut motility and reduce transit time.
Administer a specific antidote
In addition to the general management of intoxication, administration of specific antidotes may be possible if the toxicity is known or suspected and an antidote is available (see Table 2). A particular consideration in the treatment of a neurological patient is the use of intravenous intralipid emulsion therapy (IVLE), also known as Intralipid. There remains debate on the exact mechanism of action of Intralipid, however in many cases of neurotoxicity the clinical signs result from lipid-soluble toxins crossing the blood-brain-barrier, and therefore using a therapy which reduces the tissue concentration of any lipid-soluble toxicant, as is the case with intralipids, should be of benefit. The risks of its use are low but include corneal lipidosis, fat overload syndrome and pancreatitis3, and it should always be administered using a filter to reduce the risk of fat emboli. Notable neurotoxins where it is of benefit include ivermectins, permethrin, tremorgenic mycotoxins and recreational drugs. Various protocols for IVLE have been described. Dosage is usually an initial IV bolus of 1.5ml/kg given over two to three minutes, followed by a CRI of 0.25ml/kg/min for 30-60 minutes. If there is a risk of fluid overload, a reduced rate of 4ml/kg/hour for four hours has been suggested3.
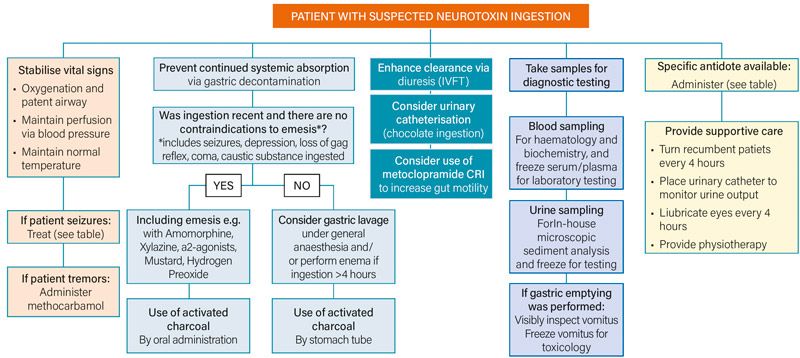
Figure 2: Flowchart diagram demonstrating the general approach to a patient with suspected neurotoxin ingestion.
Providing supportive care
Patients may present with severe neurological signs including altered mentation (obtundation, stuporous or comatose), or require sedation and/or mechanical ventilation. Intensive nursing of the recumbent patient may be required and it plays a vital role in the recovery of the patient and prevention of further complications.
Specific considerations:
If intubation is required e.g., in comatose patients, a cuffed endotracheal tube should be used. For prolonged periods of intubation, the cuff should be deflated, repositioned and then re-inflated to prevent damage to the mucosal lining due to prolonged pressure. The endotracheal tube and the oropharynx should be suctioned regularly. The tongue should be moistened regularly to prevent drying and ulceration.
Normovolaemia should be maintained and hydration corrected. Consider any fluid losses, including vomiting/diarrhoea, the effect of administered drugs (diuresis by Mannitol or hypertonic saline) and prolonged anaesthesia with subsequent free water loss from respiratory mucosa (particularly when the ventilator circuit is not humidified).
Normothermia should be maintained. Aggressive active cooling should be initiated if the patient is hyperthermic (rectal temperature greater than 40°C/104°F) until the rectal temperature is 39.5°C/103.5°F. Cooling via convection is the most effective way (wetting animal or use of cool fan). Use of ice packs can cause cutaneous vasoconstriction and slow cooling. Wet towels will impair evaporative losses. Patients with hypothermia should be slowly corrected with frequent reassessment of temperature. It is important to continually monitor circulation parameters (heart rate/rhythm, blood pressure) as well as metabolic state (particularly blood glucose concentration). Warming is most effective via circulating warm air blankets.
Patients that have ingested a caustic substance or are at risk of gastrointestinal mucosal damage or oesophagitis will likely benefit from gastro-protectant treatment. Commonly used medications include omeprazole (1mg/kg IV/PO) or sucralfate (500mg/dog q6-8hours PO (up to 20kg), 1-2g/dog q6-8 hours (>20 kg), 250mg/cat q8-12 hours PO)2. Anti-emetics may also be considered such as maropitant (1 mg/kg IV/SC) or metoclopramide (0.5 mg/kg IV/IM/SC).
General considerations
The patients should be turned every four hours to prevent pressure sores, and padded bedding provided.
Patients should be supported in sternal recumbency with regular oxygenation monitoring. There is a high risk for atelectasis leading to hypoxaemia if in lateral recumbency for prolonged periods of time.
Daily physiotherapy should be performed such as passive range of motion of the limbs.
The bladder must be effectively managed either by regular expression or catheterisation. If the latter, aseptic technique should be used with a closed system. Urinary output can be accurately calculated, and should aim for between 1-2ml/kg/hr.
Patients with impaired ability to blink will require ocular lubricants applied at least every four hours to prevent complications such as corneal ulceration from prolonged exposure.
Conclusion
A logical approach should be taken to manage any intoxicated patient, with particular consideration to those patients presenting with specific neurological signs such as tremors, mentation changes, and seizures. Prompt management of life-threatening complications such as status epilepticus and elevated intracranial pressure must be initiated, and steps taken to prevent secondary complications. Recovery can be variable, however would expect to see signs of improvement within one to two days. A full recovery is possible despite severe clinical presentation. Often those patients with acute intoxication can be the most challenging and require intensive nursing care, but they can also be the most rewarding.
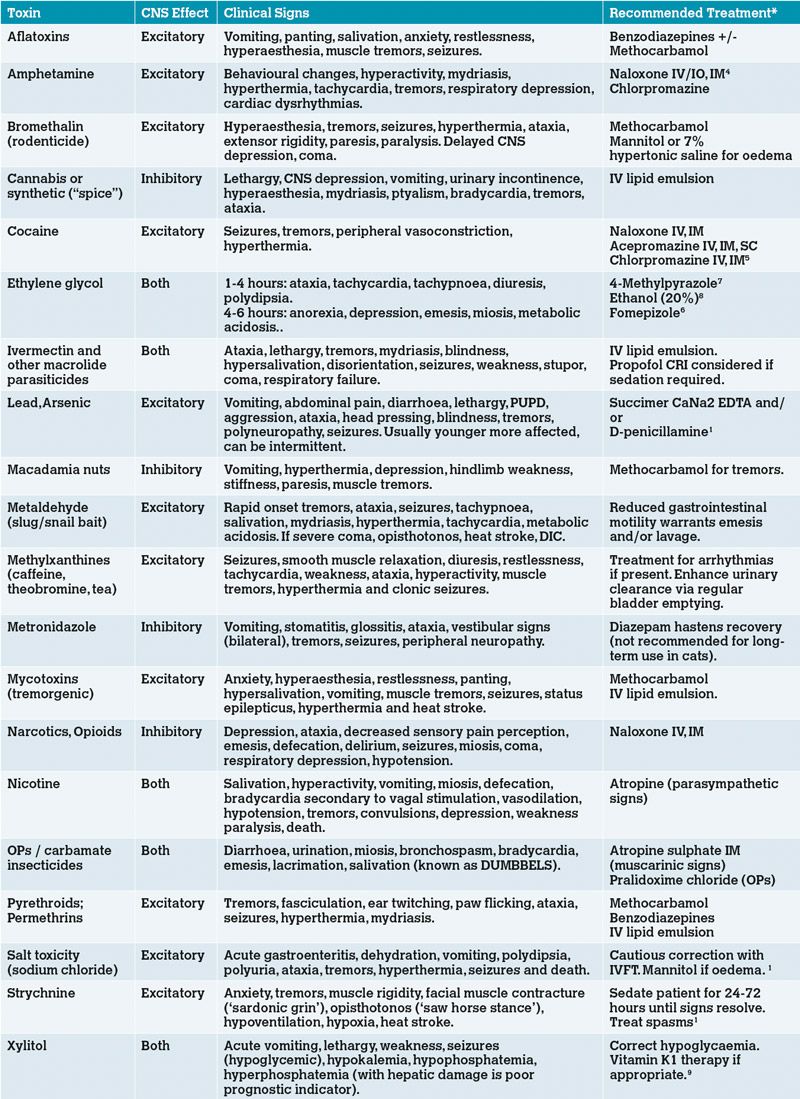
Table 2: Commonly encountered neurotoxins, their associated clinical signs and specific treatment/antidote if available. *For doses, please refer to emergency texts.
A version of this article was first published in Veterinary Practice (https://www.veterinary-practice.com/article/approaching-the-acute-encephalopathic-patient).
Acknowledgments:
A version of this paper was first published in Veterinary Practice (August 2020). An edited version is republished with permission of the editor. The author would also like to thank Dr Natalie West, Dr Danielle Whittaker, Dr Abbe Crawford and Professor Clare Rusbridge for their review of the article.
- Platt SR, Garosi LS. Small Animal Neurological Emergencies. 2012.
- BSAVA Small Animal Formulary, 9th edition, Part A. 2017.
- Robben and Dijkman 2017. Lipid therapy for intoxications. Vet Clin North Am Small Anim Pract 2017 Mar;47(2):435-450. doi: 10.1016/j.cvsm.2016.10.018. Epub 2016 Dec 21.
- Wahler BM, Lerche P, Ricco Pereira CH, Bednarski RM, KuKanich B, Lakritz J, et al. Pharmacokinetics and pharmacodynamics of intranasal and intravenous naloxone hydrochloride administration in healthy dogs. Am J Vet Res 2019; 80:696–701. https://doi.org/10.2460/ajvr.80.7.696.
- Kirk’s Current Veterinary Therapy XV - E-Book - John D. Bonagura, David C. Twedt - Google Books n.d. https://books.google.co.uk/books?id=KlJKAgAAQBAJ&pg=PA113&lpg=PA113&dq=CANINE+TOXICITY++chlorpromazine+(0.5–1+mg/kg,+IV+or+IM&source=bl&ots=XyKn93iZ7f&sig=ACfU3U0V1CRVyRDUGhAESNLpY6FejYleGw&hl=en&sa=X&ved=2ahUKEwjqmNf0lsPpAhXGQhUIHd9mCYMQ6AEwDnoECAoQAQ#v=onepage&q=CANINE TOXICITYchlorpromazine(0.5–1 mg%2Fkg%2CIVorIM&f=false (accessed May 20, 2020).
- Connally HE, Thrall MA, Hamar DW. Safety and efficacy of high-dose fomepizole compared with ethanol as therapy for ethylene glycol intoxication in cats. J Vet Emerg Crit Care 2010;20:191–206. https://doi.org/10.1111/j.1476-4431.2009.00492.x.
- Tart KM, Powell LL. 4-Methylpyrazole as a treatment in naturally occurring ethylene glycol intoxication in cats. J Vet Emerg Crit Care 2011;21:268–72. https://doi.org/10.1111/j.1476-4431.2011.00638.x.
- Barceloux DG, Krenzelok EP, Olson K, Watson W, Miller H. American academy of clinical toxicology practice guidelines on the treatment of ethylene glycol poisoning. J Toxicol - Clin Toxicol 1999;37:537. https://doi.org/10.1081/CLT-100102445.
- 9. Murphy LA, Coleman AE. Xylitol Toxicosis in Dogs. Vet Clin North Am - Small Anim Pract 2012;42:307–12. https://doi.org/10.1016/j.cvsm.2011.12.003.
1. What three clinical features are suggestive of Cushing’s Reflex in cases of severe elevated intra-cranial pressure?
A. Tachycardia, hypertension, altered breathing pattern
B. Bradycardia, hypertension, altered breathing pattern
C. Bradycardia, hypotension, seizures
D. Tachycardia, hypotension, seizures
2. What is NOT a contraindication for inducing emesis?
A. Altered mentation
B. Loss of gag reflex
C. Ingestion of caustic substances
D. Ingestion within 2 hours
3. Which of the below specific therapies is indicated in the treatment of ivermectin, permethrin, tremorgenic mycotoxins and recreational drug toxicity?
A. Intravenous intralipid therapy
B. Ethanol
C. Atropine
D. Succimer CaNa2 EDTA
4. Which of the below toxins typically causes signs of central vestibular disease?
A. Ethylene glycol
B. Metaldehyde
C. Botulism
D. Metronidazole
5. Which medication is typically indicated for treatment of tremors?
A. Levetiracetam
B. Mannitol
C. Methocarbamol
D. Phenobarbitone
Answers: 1B; 2D; 3A; 4D; 5C.









