National genotyping programme: a guide for vets
The National Genotyping Programme (NGP) aims to enable Ireland take the initial steps in becoming the first country in the world to genotype its national herd. It will also contribute to Ireland’s objective to be a world leader in sustainable food systems, which is in line with our shared ‘Food Vision 2030’ Strategy. Doreen Corridan MVB MRCVS, CEO of the National Cattle Breeding Centre which runs dairy and beef breeding programmes for Munster Bovine and Progressive Genetics, provides an overview of the programme
NGP is voluntary, is available to beef herd owners and dairy herd owners, pedigree and/or commercial, and will run over a five-year period. Ireland has made a good start on genotyping, particularly through the Beef Data and Genomics Programme (BDGP) and its successor the Suckler Carbon Efficiency Programme (SCEP) and those herds that have taken part in the NGP in 2023 and 2024.
Genotyping the national herd will provide a huge opportunity for both the dairy industry and the beef industry to accelerate gains in our national breeding indexes (e.g., EBI, Eurostar, and DBI) which will enhance farm sustainability.
Veterinary surgeons should encourage their dairy and beef breeding herd owners to join the National Genotyping Programme (NGP) now.
What will NGP deliver for vets?
The NGP is designed to deliver value for veterinarians, for farmers and for industry groups. For veterinarians, NGP will assist in integrating genetics into herd health and production planning for herd owners. For farmers, the NGP will deliver improved herd performance, profitability, and sustainability. For industry bodies, the NGP will enhance national herd quality, genetic merit, traceability, and climate resilience.

Figure 1: Example of DNA registration timeline.
NGP: funding and pricing
NGP is run through a strategic public-private partnership. A total investment of €43 million, over five years, is committed by the Department of Agriculture, Food and the Marine (DAFM). The NGP is managed by the Irish Cattle and Breeding Federation (ICBF) on behalf of the DAFM. The first year of the programme (2023) was funded from the Brexit Adjustment Reserve (i.e., government-funded), with a budget of €23 million. From 2024 onwards, NGP operates on a cost-sharing model between the DAFM (providing one third of the funding), Dairy Industry Ireland (DII) and Meat Industry Ireland (MII) (providing one third of the funding), and participating farmers (providing one third of the funding).
To participate, farmers must commit to the full five years of the NGP. Initially, herd owners get all their breeding females and stock bulls genotyped free of charge. Subsequently (years two to five), the cost to herd owners is €6 per calf born, i.e., €4 to genotype plus €2 for the costs of the double tissue tag and postage. The remaining cost (€18 - €6 = €12) will be shared equally between the DAFM, and DII & MII.
This price model means heavily subsidised genomic testing rates for farmers (i.e., €6 per animal). In the UK, US, or New Zealand full genotyping can exceed €60–€80 per animal.
By making genomic testing widely available and affordable, the programme offers powerful tools for improving genetic accuracy, herd health, profitability, and environmental performance. Backed by strong industry support and public funding, Ireland’s NGP is delivering world-class genetic technology to commercial farms at a fraction of the global cost. This places Irish farmers at the forefront of innovation in livestock breeding, traceability, and sustainability, as well as contributing to Ireland’s climate action targets.
How does genotyping work?
Genotyping involves taking a tissue sample from breeding females and males and building a DNA bank of that data. When new calves are born, they are matched to their sire and dam. The genetic data collected will be analysed to identify specific traits or characteristics that are important to farmers – this includes disease resistance, milk or meat production, or fertility. In addition, the information gathered will be used to develop breeding strategies aimed at improving the overall genetic merit of the herd. If carried out at a national scale, genotyping would give the Irish cattle industry 100 per cent traceability at the DNA level.
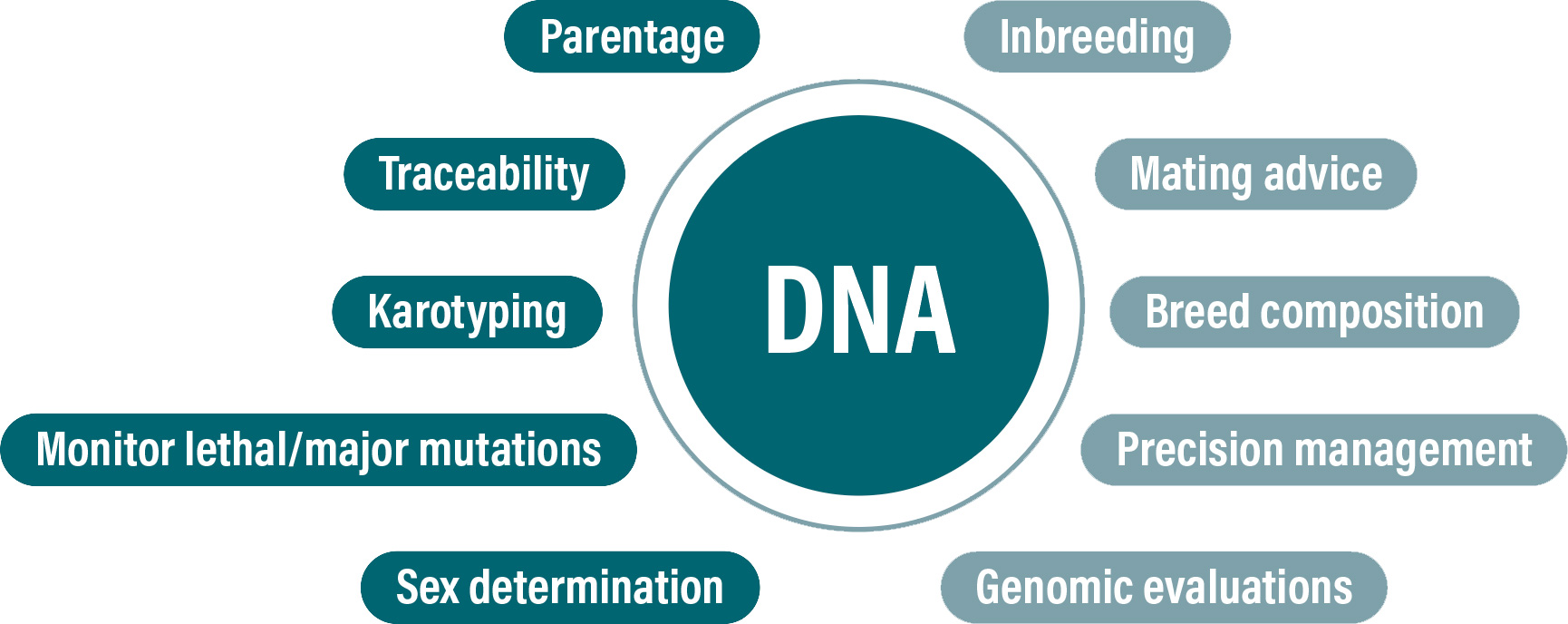
Figure 2: Potential uses of DNA information in cattle.
What’s involved for herd owners?
Preparation: Herds who sign up in 2025 will receive button tags to DNA-sample all their breeding females and stock bulls that are not already genotyped. There is no cost to the herd owner in genotyping these animals. Tissue tags, similar to the BVD sampling tag, will be issued and herd owners will have 28 days to return samples. All ancestry will be verified: sires will be allocated where possible and corrected. Having all the breeding females sampled in advance of the 2026 calving season ensures that the online DNA registration process works effectively and efficiently, i.e., the DNA of a calf born in 2026 will match up correctly to its corresponding dam.
Ordering Tags: To avoid any potential issues around tag types for 2026, farmers, who are accepted into the Programme, can only order the specific ‘Double Tissue Tags’ from their chosen tag supplier. So, one sample for the BVD programme and another sample for the NGP programme. All tags must be ordered before the 2026 calving season.
DNA Calf Registration: All farmers who avail of the free genotyping in 2025 are then committed to registering their calves via the appropriate DNA registration channels. ALL calves on the holding must be registered via the official DNA registration process.
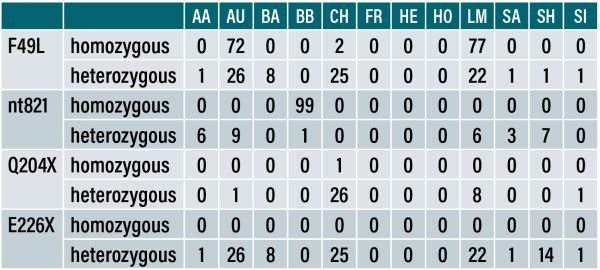
Table 1: The percentage of purebred animals carrying at least one mutant allele for each of the common myostatin variants.
Benefits of NGP for the herdowner
Parentage Verification: With the NGP programme, genotyping at birth will confirm parentage of the dam and the sire, as well as correcting any errors prior to calf registration and issuing of the passport. Parentage errors (which currently sit at an average of 15 per cent nationally) lead to incorrect breeding values for both dairy and suckler herd owners and create paperwork to correct the passport once the animal is already registered. By herd owners’ sampling their cows, it will correct any past parentage mistakes in the herd, and this will give the confidence that inbreeding will be avoided going forward.
Every calf will be registered with the correct mother: If a herd owner has a number of cows calved one night and does not know which calf belongs to which mother, with the NGP there are no worries. This is because the ear sample taken from the calf will tell with 100 per cent accuracy whose mother it is. The herd owner just needs to record the date of birth. In addition, this will ensure replacements are kept from the herd owners’ best cows.
Every calf will be registered to the correct AI sire or stock bull: Regardless of how many stock bulls are running with the cows or heifers and for what period, the calves from each stock bull will be identified. This will clearly identify the most fertile stock bull, or the infertile bull. In addition, it will identify the bull that is producing the best quality calves for sale. It will allow the identification of easy calving and difficult calving stock bulls.
Every calf will be registered to the correct AI sire: So, no worries if the cow may have held to a previous insemination. Ancestry data will be correct in the herd, and this will link with the AI technician’s handheld when cows are being inseminated. This will not only ensure that replacements are kept from the best cows but also that the sale price of calves is maximised. This is especially important if elite AI sires are being used, as now the calves will be verified to the correct sire.
Labour savings and simpler calf registration: Farmers who sign up to the NGP will have exclusive access to double tissue tags. By sampling animals at birth, farmers don’t have to wait for button tags or hair-cards for SCEP or Pedigree Societies and then round up and bring in animals for sampling at a later stage. It is also much safer and easier to tag and sample newborn animals than more mature animals. Secondly, there is no extra paperwork in correcting existing passports as now the corrections are made prior to the passport being issued. The sire, dam, and sex can be determined from the DNA sample.
Graph 2. Calving difficulty index of AI bulls comparing non-carriers to heterozygous for nt821 and homozygous for nt821.
Genomic prediction of genetic merit: One of the NGP’s most impactful features is the early-life prediction of genetic merit through genomic breeding values. This will now be much more accurate due to the correct parentage and due to the DNA analysis. These values are generated using the animal’s DNA and include traits relevant to the dairy herd owner such as: milk yield and solids, fertility and calving interval, health and longevity, and resilience to TB. This information is available within three weeks of the birth of the calf.
This enables decisions to be made immediately on which calves are genetically superior at a very young age to select as replacements. This means reduced costs and labour of rearing lower genetic merit animals, thus leading to greater long-term herd productivity.
All calves born in herds in the NGP will receive a CBV value which shows the commercial beef value of the calf in euros as a beef animal. This will ensure herd owners get a just price for their calf based on its genetic potential. This rewards dairy and beef herd owners using beef AI and good quality beef stock bulls. The number of herds rearing dairy beef calves has increased with the increasing supply due to the plateauing of the dairy herd and the increased use of sexed semen.
Breed confirmation: Whether breeding for milk, beef, or crossbred performance, knowing the true breed composition of each animal helps optimise outcomes. Herd owners can match breeding programmes to market demands. Veterinarians can better understand breed-related health or calving risks. Industry stakeholders gain more consistent data for benchmarking. Breed confirmation is particularly useful in beef-on-dairy systems, where balancing calving ease and carcase value is critical.
Management of deleterious genetic defects: Genotyping plays a critical role in identifying carriers of harmful genetic mutations – such as Holstein Lethal Haplotypes (HH1–HH6) – associated with early embryonic death and reduced fertility. Myostatin variants found in beef breeds can impact calving ease and muscular development. By identifying carrier animals early, veterinarians and herd owners can avoid risky matings and phase out undesirable genes over time thus improving welfare and herd productivity.
Reduction in carbon emissions: Improved genetics contribute directly to lower emissions intensity. Genetically superior animals produce more milk or meat with fewer inputs, reach production targets more efficiently, have improved fertility and health, and reduce waste. These outcomes reduce methane, nitrogen, and carbon emissions per kg of output. As Ireland moves toward its Climate Action Plan targets, the NGP supports the livestock sector’s contribution to national emissions reductions.
Genocells technology: Genocells allows dairy herd owners to determine each cow’s contribution to the bulk tank somatic cell count using a single bulk tank milk sample. Herd owners are finding this service extremely convenient especially if they experience a spike in SCC in between milk recordings. However, to operate effectively, the Genocells technology needs all the cows to be genotyped. The NGP provides a natural pathway for this to happen, i.e., the rollout of this new service to Irish dairy herd owners.
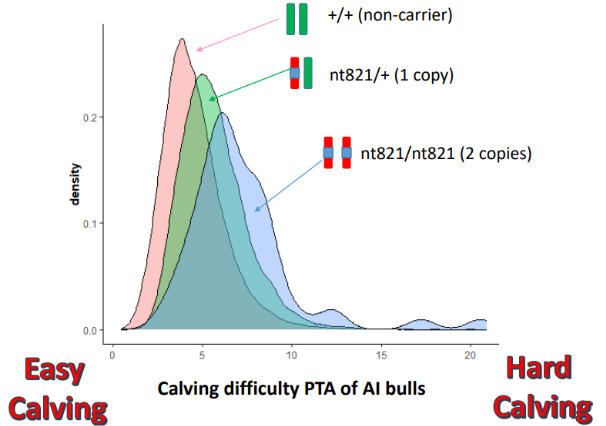
Graph 2. Calving difficulty index of AI bulls comparing non-carriers to heterozygous for nt821 and homozygous for nt821.
Benefits of NGP for the vet
Fertility analysis pre-breeding season: From a veterinarian’s point of view, the NGP will improve the accuracy of fertility analysis. It will clarify the conception to each insemination sexed or conventional in both cows and heifers and clarify the performance of stock bulls. This will ensure a more accurate breeding plan is implemented for the subsequent breeding season.
Genomic prediction of genetic merit: The investigation of poor performance in herds can be difficult without accurate knowledge of the herd’s genetic potential. From a veterinarian’s point of view, genomics will allow the calculation of the genetic potential of the herd accurately. In addition, it allows the veterinarian to break it down to the performance of each lactation group and ensure that the herd maximises its genetic potential through management and appropriate nutrition. This will improve the outcomes of the veterinarian’s recommendations. Confirmation of the breed composition will also aid in the investigation of poor performance; an example would be where Angus crosses are unknowingly being milked in cross-bred herds.
All calves born in herds in the NGP will receive a commercial beef value (CBV) which shows the commercial beef value of the calf in euros as a beef animal. This will ensure herd owners get a just price for their calf based on its genetic potential. From a veterinarian point of view, the profitability of dairy calf to beef herds is dependent on performance and the value of the carcase obtained – purchasing calves with a high CBV will ensure these herds have the potential to deliver a profitable enterprise. In veterinary practices, the private veterinary practitioner (PVP) is in the unique position of being able to advise the dairy herd owner on which sires to use and the calf finisher on which calves to purchase to enable a profitable enterprise. The accurate information available from genotyping will also contribute to enhanced integration between the dairy and beef sectors, and to improving animal health and welfare.
Precision breeding tools: Genomic data enhances the ICBF sire advice tool that optimises breeding decisions for dairy females. Genomic data assists in recommending sires that improve production, fertility, and health including TB resilience. It avoids inbreeding or pairing of carriers of genetic disorders and supports targeted breeding for key economic traits. Veterinarians can use this data to support breeding consultations, and to assist herd owners to make sustainable and welfare-conscious mating decisions.
Decision making in relation to calving: Myostatin variants found in beef breeds can impact calving ease and muscular development. By identifying carrier animals early, veterinarians and herd owners can avoid risky matings and phase out undesirable genes over time thus improving welfare and herd productivity. When presented with a calving cow, knowing the myostatin variants of both the dam and sire is extremely valuable information to maximise successful outcomes.
SCC control and response to treatment: Genocells is an extremely useful technology in identifying the highest percentage contributors to the bulk tank SCC. In between milk recordings, it is very convenient to just take a bulk tank sample and identify the cows contributing to a spike in the bulk tank or to measure the response to treatment of a group of cows treated after a milk recording. At the back end of the year after the last milk recording when the final group of cows are being dried off it will help with the decision to use sealer only or to treat individual cows.
Personalised veterinary medicine: Genomic data allows for targeted health management strategies, including tailored vaccination plans and antimicrobial stewardship, based on an animal’s/herd’s unique genetic makeup.
What is myostatin?
The myostatin gene (GDF8) is found in all mammals and influences the production of a protein that controls muscle development. When the gene functions correctly, muscle growth is controlled, resulting in normal-sized animals. However, when mutations (or physical breaks) occur in the myostatin gene, muscle growth accelerates, producing more muscled animals, often referred to as ‘double-muscled’ animals.
There are 21 known myostatin mutations (variants) of the myostatin gene in cattle, some of which are breed-specific and others which affect more than one breed.
The mutations in the myostatin gene can vary in their effects. A partial break in the gene (missense), such as the F94L mutation, is commonly found in Limousin cattle. Animals with this mutation typically show improved carcase traits without any impact on calving difficulty.
On the other hand, full breaks in the myostatin gene (disruptive) – such as the Q204X mutation, which is mainly found in Charolais, the nt821 mutation mainly found in Belgian Blues, and the E226X mutation in Shorthorns – can lead to significant increases in muscle mass. While these mutations enhance carcase traits, the nt821 and Q204X mutations are also associated with increased calving difficulty.
Of the nine most common variants, six are classified as disruptive – nt821, Q204X, nt419, E226X, C313Y, and E291X. Three are referred to as non-disruptive – S105C, F94L, and D182N.
A proportion of animals from most of the major breeds in Ireland (Angus, Aubrac, Belgian Blue, Blonde, Charolais, Limousin, Salers, Shorthorn, Parthenaise, Piedmontese and Simmental) carry a mutation of the myostatin gene and, consequently, these animals show hereditary double-muscling.
The six disruptive myostatin genes cause muscle hypertrophy (double-muscling) in cattle, but are also associated with larger birth weights, increased dystocia and enhanced tenderness.
The three non-disruptive myostatin genes will increase muscularity, but not cause double-muscling, and reduce external and intramuscular fat, with no change in birth weight.
How myostatin is inherited
Cattle inherit one copy of each myostatin mutation from each parent. So, for each myostatin mutation, every animal can either have:
- no mutations (two working copies of the gene) – normal muscling;
- one mutation (one working copy, one mutated) – intermediate muscling; or,
- two mutations (both copies mutated) – double muscling.
Animals with one copy of a mutation have a 50 per cent chance of passing that mutation onto their calves; animals with two copies of a mutation will always pass one copy of the mutation onto their calves. That’s why it’s important to know the genetics of both the bull and the cow when breeding.
For example, if a bull has two copies of the nt821 mutation and the cow has none, the calf will inherit one mutated and one normal gene. These calves tend to have better muscling without the extreme calving risk. But if both parents pass on nt821, the calf will have two mutated copies, leading to extreme muscling and a much higher risk of calving difficulty.
Myostatin and carcase traits
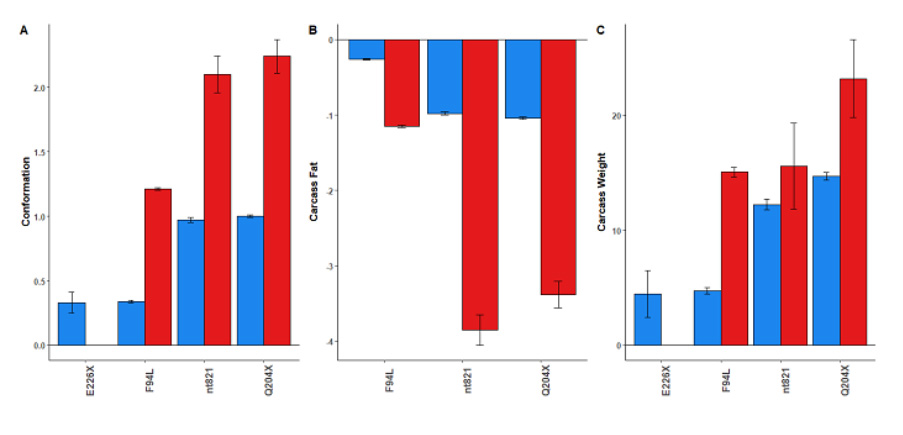
While myostatin mutations can positively affect carcase weight, fat distribution, and conformation, the impact varies depending on whether animals carry one or two copies of the mutation. Typically, animals with no copies of the mutation are lighter with slightly fatter carcases and poorer conformation. Those with one copy show improved traits, and animals with two copies see further improvements in carcase traits. Graph 1 quantifies the improvement in carcase traits that animals carrying one or two copies of the myostatin mutations on average have, relative to animals with no copies of the myostatin mutations.
Myostatin and calving difficulty
Research carried out by Cliona Ryan, Teagasc showed that the presence of F94L, nt821 and Q204x were all associated with increased incidence of calving difficulty when present in either the dam or the calf, and nt821 had the greatest observed impact on calving difficulty when present in either the progeny or the dam (see Graph 2).
The importance of understanding myostatin status
Despite the production positives, not all myostatin mutations are suitable for every beef system. It is essential for farmers to understand the potential for increased calving difficulty associated with mutations like nt821 and Q204X.
It is advisable that herd owners establish the myostatin status of both bulls and cows before breeding to avoid complications like calving difficulty. This is available on the ICBF HerdPlus app when an animal is genotyped. Cows that carry two copies of mutations, such as nt821 or Q204X, may have narrower pelvises, increasing the incidence of dystocia. This risk of dystocia is significantly increased if they are bred to a bull that also carries two copies of the same mutation, as the resulting calf will be double-muscled, making it harder to deliver through an already restricted birth canal. This is extremely useful information for veterinarians which will lead to better outcomes, and more informed decision-making on whether to perform a caesarean section or not, when presented with a cow calving and not making progress.
Genocells
Genocells is a bulk milk tank test to determine the SCC in genomic-tested herds. It allows dairy herd owners to determine each cow’s contribution to the bulk tank’s somatic cell count using a single bulk tank milk sample. In association with ICBF, Munster Bovine and Progressive Genetics (two leading Irish companies providing AI and herd management services for dairy and beef farmers) have brought the groundbreaking Genocells technology to the Irish market. This service is available to milk recording herd owners who have their herds genotyped. Herd owners are finding this service extremely convenient, especially if they experience a spike in SCC in between milk recordings. However, for the Genocells technology to operate effectively it needs all the cows in the herd to be genotyped. The NGP provides a natural pathway for this to happen, i.e., the rollout of this new service to Irish dairy herd owners.
How does Genocells technology calculate SCC?
GenoCells determines with high reliability the somatic cells count from the DNA analysis of a sample of milk. Genocells works by extracting the DNA from the somatic cells in the milk and comparing the genotype of this extracted DNA to the recorded genotype of the cows contributing milk to the bulk tank. As somatic cells are the only milk components that contain DNA, the percentage of DNA that each cow contributes to the overall DNA volume in the bulk tank is equivalent to the percentage of somatic cells that each cow contributes to bulk tank SCC. This is known as percentage cellular responsibility.
Individual SCCs are then calculated using the percentage cellular responsibility, the percentage of the milk volume that the cow contributed to the tank (calculated using milk recording yields) and the bulk tank SCC result.
Munster Bovine, Progressive Genetics, and ICBF undertook a comprehensive pilot study in July/August of 2024 where 85 herds submitted three samples each over a number of weeks to validate the following:
accuracy of Genocells: the test consistently identified the highest SCC cows with an accuracy rate ranging from 90-98 per cent, confirming the reliability of Genocells in detecting high SCC contributors; and,
logistical turnaround time: the time from farm sample collection to receiving results was consistently within 7 days, ensuring a quick and efficient service.
At the conclusion of the trial, a survey was conducted to gather participants’ feedback on the Genocells programme. The results were overwhelmingly positive: 93 per cent of respondents saw value in using Genocells alongside their existing milk recording practices. In all, 97 per cent of respondents would recommend Genocells to other farmers.
Who is Genocells technology suitable for?
Genocells will provide more points of information over and above the four milk recordings to manage herds with high or fluctuating cell counts. Genocells is also suitable for low cell count herds that experience spikes in cell count as it is a simple and convenient way of identifying high SCC cows relatively quickly. Genocells can also provide more points of information about individual cows before drying off to help make better decisions regarding dry cow therapy and cow retention.
Conclusion
The NGP is not just a technological upgrade, it is a national strategy to build a more profitable, traceable, and sustainable livestock industry. With benefits ranging from parentage verification to climate-smart genetics, the NGP empowers farmers and vets with actionable data that leads to better decisions, healthier animals, and stronger returns.
Cliona A Ryan, Deirdre C Purfield, Saeid Naderi, Donagh P Berry, Associations between polymorphisms in the myostatin gene with calving difficulty and carcass merit in cattle, Journal of Animal Science, Volume 101, 2023, skad371, https://doi.org/10.1093/jas/skad371
Berry DP, Spangler ML, The Benefit of a National Genomic Testing Scheme. Vet Clin North Am Food Anim Pract. 2024 Nov;40(3):435-445. doi: 10.1016/j.cvfa.2024.05.008. Epub 2024 Jul 8. PMID: 38971688.
D.P. Berry, M.L. Spangler, Animal board invited review: Practical applications of genomic information in livestock, animal, Volume 17, Issue 11, 2023, 100996, ISSN 1751-7311, https://doi.org/10.1016/j.animal.2023.100996.
P. Lenormand, F. Perrin, M. Calmels, V. Charrier, M. Foucher, J.B. Davière. Editors: J. Kucera, P. Bucek, D. Lipovsky, X. Bourrigan, M. Burke. GenoCells: individual somatic cell count of dairy cows by genotyping tank milk. Bulletin article (Conference paper): ICAR Technical Series, 2019, No. 24, 107-110 ref. 2 ref. Conference title: 43rd ICAR Conference, Prague, Czech Republic, 17-21 June 2019.
Blard, G., Z. Zhang, W. Coppieters and M. Georges 2012. Identifying cows with subclinical mastitis by bulk single nucleotide polymorphism genotyping of tank milk. J. Dairy Sci. 95: 4109-4113.
Perrin, F. and Marg-Haufe B., 2019. A revolution in milk sample analysis. TECAN Journal 1/19, 12-13.
1. What does NGP stand for?
- Natural Growth Programme
- National Grazing Programme
- National Genetics Programme
- National Genotyping Programme
2. What is GenoCells?
- Determines the somatic cell count from the DNA analysis of a bulk milk sample
- A vaccine used to boost milk yield in dairy cows
- A feed additive to enhance fertility in cattle
- A genetic test used only for identifying calf gender
3. What does the Myostatin gene (GDF8) influence?
- The production of a protein that controls muscle development
- The marbling quality of meat
- The fertility rate in cows and heifers
- The ability of animals to convert muscle to fat
4. What does CBV stand for?
- Calving Birth Weight Value
- Certified Breeding Value
- Commercial Beef Value
- Carcase Balance Volume
ANSWERS: 1D; 2A; 3A; 4C.






