The role of bulk tank milk testing in monitoring BVD and IBR
Dr Maria Guelbenzu LV PhD MRCVS, BVD and IBR Programme Manager, AHI, discusses the use of bulk tank milk testing to monitor BVD and IBR
The detection of antibodies is a useful and cost-effective tool for the monitoring and control of diseases. The application of bulk tank milk (BTM) as a herd level diagnostic tool has been used extensively in bovine viral diarrhoea (BVD) and infectious bovine rhinotracheitis (IBR) disease control programmes around Europe (Greiser-Wilke et al, 2003; Raaperi et al, 2014). This collection of samples is cheap and easy, helping to reduce the cost of surveillance programmes.
Some limitations are also recognised including the dilution factor due to sample pooling and the fluctuation in the level of protein, including antibodies, in milk during the lactation cycle, which can effectively increase or decrease the dilution ratio.
Since 2019, DAFM has undertaken national surveillance of dairy herds for BVD and IBR gE antibodies using bulk tank milk samples, with two rounds of testing per year being carried out each spring and autumn. The results from this testing will shortly be made available to herd owners through ICBF. Interpretation of these results will be discussed in this article. It is envisaged that BVD BTM will be used as part of the post-freedom surveillance strategy of the programme, when a large proportion of herds will be seronegative. The communication of the IBR BTM results will be accompanied by targeted messaging in advance of any decision on a national programme. This will encourage measures to reduce IBR prevalence, consistent with one of the Animal Health Actions contained within the AgClimatise Roadmap and the Annex to the Climate Action Plan (CAP) published in 2021 ( CAP: https://www.gov.ie/en/publication/6223e-climate-action-plan-2021/; AgClimatise: www.gov.ie/en/publication/07fbe-ag-climatise-a-roadmap-towards-climate-neutrality/).
Testing for BVD antibodies
Two distinct approaches are applied to the diagnosis of BVD, identifying either the virus itself or detecting immunity to it. The Irish BVD programme has until now focused on the detection of virus in young calves. However, the post-freedom phase will be aimed at herd level surveillance, which is commonly performed with antibody detection methods.
The detection of antibodies to BVDV is a useful tool for determining an animal’s immune status and previous exposure. A positive result in an unvaccinated animal will suggest exposure, with immunity generally considered to be life-long. In a pregnant animal, it also indicates the possibility that the dam is carrying a PI animal. A negative antibody result doesn’t confirm that the animal is naïve as it can also be persistently infected. At this stage in the programme this is highly unlikely, but an antigen/viral detection test is required to exclude this possibility. In samples from persistently infected (PI) calves, BVDV-specific maternal antibodies may be detected and may also block the detection of viral antigens, for up to 75 days, leading to false negative results. In non-PI calves, colostral antibodies last longer and can be detected until the animals are around six to nine months old, depending on the quality of the colostrum and the amount received by the calf.
Antibody testing also can be used for the detection of transient/acute infection and, at herd level, for herd classification or monitoring within control programmes by testing bulk milk for antibodies or by testing a proportion of the youngest age cohort of animals that are old enough not to have maternally derived antibodies for BVDV antibodies (Check test).
Types of BVD antibody tests
The ELISA is the most common technique utilised in diagnostic laboratories for antibody detection. A wide number of kits are available on the market which fall into two main types: kits detecting antibodies against the whole virus, which tend to be of the indirect format and kits targeting antibodies raised to the p80 (also referred to as NS2/3) protein only, which tend to be of the competitive (or blocking) format.
Indirect kits have been used within the Scandinavian disease control programmes to classify and monitor herds, primarily with bulk milk but also used when testing young stock groups (first lactation and spot/check tests). They detect antibodies derived from natural infection as well as from vaccination (González et al, 2014).
Non-structural proteins, such as p80 are mainly produced during viral replication, so cattle are more likely to develop antibodies to these after natural infection than after vaccination with an inactivated vaccine (Graham et al, 2003; González et al, 2014).
Both these formats of kits will give positive results in animals that have been vaccinated with live vaccine. Also, both can generate positive results in animals vaccinated with inactivated vaccines, although this is generally considered less likely with competitive (p80) than indirect (whole virus) kits.
Interpretation of BVD BTM results
A positive BVD BTM test result is obtained in herds with moderate to high prevalence of seropositive animals. Seropositivity could arise after infection, vaccination or both. In contrast, a very low or negative value indicates that that there are no, or a small proportion of, milking animals that have been exposed to the BVD virus.
As mentioned above, vaccines can generate positive results in the absence of infection. The recent administration of inactivated vaccine may cause a temporary increase in the BTM antibody levels. With BVD, animals exposed to the field virus or to modified-live vaccines will be seropositive for a long period of time (considered life-long). Therefore, the vaccination history is key for the interpretation of serological results for BVD.
Regular bulk tank milk (BTM) testing can be used in test negative herds to monitor the milking herd’s disease status. Antibody levels in the bulk milk will become detectable or increase if the BVD virus starts spreading within the milking herd.
Testing for IBR antibodies
The detection of antibodies to bovine herpes virus-1 (BoHV-1) is an important tool for the control and diagnosis of IBR. Once an animal becomes infected with BoHV-1, the virus becomes latent and the animal remains infected for life. These animals develop antibodies. Therefore, the identification of serologically positive animals provides a useful and reliable indicator of infection status. Any animal with antibodies to the virus is considered to be a carrier and a potential intermittent excretor of the virus. The only exceptions are calves with maternally derived antibodies from their dam, and non-infected cattle vaccinated with non-marker vaccines. Only non-marker vaccines have been on the market in Ireland for a considerable period, but both marker and non-marker vaccines continue to be used in Northern Ireland.
Types of IBR antibody tests
As with BVD, the ELISA is the more common technique applied. Two main types of ELISA tests for the detection of BoHV-1 antibodies exist: conventional and marker tests. Within the conventional kits, we again have those targeting the whole virus, which tend to be of the indirect format and kits detecting antibodies raised against the gB protein of the virus, which tend to be of the competitive format. Both these formats of conventional kits will give positive results in animals that have been vaccinated in addition to animals that have been infected.
Currently, the only available serological marker tests are the BoHV-1 gE-antibody blocking ELISAs. Animals vaccinated with gE-deleted marker vaccines can be discriminated from field-virus infected animals by a negative serological reaction for gE (Table 1).
gE-ELISAs have been shown to be less sensitive and specific than the conventional ELISAs. In addition, seroconversion against gE can be delayed when applied to individual animals, especially in vaccinated animals, to between day 21 to 35 post infection. However, they have been applied successfully before in control and eradication programmes in other countries.
Interpretation of IBR BTM results
When testing bulk tank milk samples, indirect ELISAs have shown the highest sensitivity and have been used extensively in non-vaccinating, Nordic countries within their IBR eradication and surveillance programmes.
The gE-blocking ELISA in bulk milk gives a positive reaction only when more than 10–15 per cent of the lactating animals contributing to the tank are infected although the sensitivity can be increased by milk concentration protocols. Therefore, this test is not typically used to declare a herd to be free from BoHV-1 infection. However, for general surveillance purposes, bulk milk tank tests can give an estimate of BoHV-1 prevalence in a herd, an area or country.
A positive IBR gE bulk tank milk (BTM) test result is obtained in herds with moderate to high prevalence of latently infected animals, with ongoing circulation of the virus. These herds should be encouraged to start carrying out complete and regular herd vaccination. Vaccination makes it less likely that a latent carrier will reactivate and shed the virus, and less likely that a naïve animal will become infected and spread the virus after exposure. In these herds, vaccination combined with bioexclusion is the most practical and appropriate control option (Figure 1). It is likely that bioexclusion and vaccination will need to be used for a period of years before herd prevalence reduces to the point where the bulk tank becomes test negative.
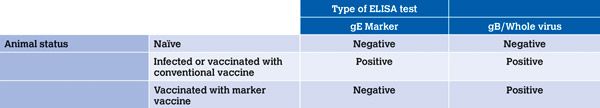
Table 1: influence of animal status and test methods on test results.
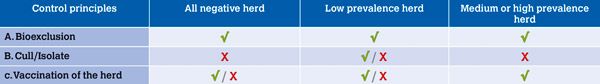
Figure 1: methods for IBR control as per within herd prevalence.
A negative IBR gE bulk tank milk (BTM) result indicates that there are no, or less than 15 per cent of, milking animals that have been exposed to the IBR virus. For vaccinating herds, this result is evidence that vaccination is working as there is no/low circulation of the virus within the milking herd. In addition, herds with repeated gE negative results are likely to be free of infection, or very close to it, and this can be used as an indication to carry out whole herd test to confirm freedom or to identify small numbers of carriers still present, with these typically being in the older cohort of animals or purchased.
Conclusions
Bulk milk samples provide a non-invasive, cost-effective way of monitoring herds for both BVD and IBR. A key piece of information in order to correctly interpret BVD results is the vaccination history. This will become less important when freedom is achieved, as routine BVD vaccination will be prohibited. Results for IBR can be used to help the herds control IBR on farm or to keep the farms disease-free and to reduce the overall prevalence of the disease in Ireland.
Within the surveillance carried out by DAFM, around 17,000 samples were tested every spring and autumn from 2019 until the spring of 2021, with the exception of spring 2020, where there were around 12,000 samples received. For IBR, the analysis of results shows no consistent decline in prevalence, with an average prevalence of 52 per cent, despite year-on-year increased vaccines sales.
In contrast, BVD results show an overall downwards trend with 50 per cent negative at most recent test round available. This is a considerable reduction when compared to 88 per cent in 2015 from an earlier study (Sayers et al, 2015). To gain a better understanding of the results available to date, and factors associated with being test-positive, a detailed analysis by the Centre for Veterinary Epidemiology and Risk Analysis (UCD) and Animal Health Ireland is under way.
- González, A. M., Arnaiz, I., Yus, E., Eiras, C., Sanjuán, M., and Diéguez, F. J. (2014). Evaluation of long-term antibody responses to two inactivated bovine viral diarrhoea virus (BVDV) vaccines. Vet. J. 199, 424–8. doi:10.1016/j.tvjl.2013.12.005.
- Graham, D. A., German, A., Mawhinney, K., and Goodall, E. A. (2003). Antibody responses of naive cattle to two inactivated bovine viral diarrhoea virus vaccines, measured by indirect and blocking ELISAs and virus neutralisation. Vet. Rec. 152, 795–800.
- Greiser-Wilke, I., Grummer, B., and Moennig, V. (2003). Bovine viral diarrhoea eradication and control programmes in Europe. Biologicals 31, 113–118. doi:10.1016/S1045-1056(03)00025-3.
- Raaperi, K., Orro, T., and Viltrop, A. (2014). Epidemiology and control of bovine herpesvirus 1 infection in Europe. Vet. J. 201, 249–256. doi:10.1016/j.tvjl.2014.05.040.
- Sayers, R. G., Byrne, N., O’Doherty, E., and Arkins, S. (2015). Prevalence of exposure to bovine viral diarrhoea virus (BVDV) and bovine herpesvirus-1 (BoHV-1) in Irish dairy herds. Res. Vet. Sci. 100, 21–30. doi:10.1016/j.rvsc.2015.02.011.
- SCAHAW (2000). SCAHAW (Scientific Committee on Animal Health and Welfare) Report on Bovine Herpes Virus 1 (BHV1) marker vaccines and the accompanying diagnostic tests.
1. A sudden increase in BVD BTM antibody levels for a herd that was previously BTM negative could mean:
A. The milking herd has been exposed to the BVD virus
B. The milking herd has recently been vaccinated with an inactivated BVD vaccine
C. The milking herd has recently been vaccinated with a live BVD vaccine
D. All of the above
2. A positive BVD antibody test (whole virus or p80) in an animal could indicate:
A. Exposure to the BVD virus
B. Maternally derived antibodies in young calves (including PI calves)
C. Foetal infection in the last trimester of pregnancy
D. Possibility of carrying a PI calf in pregnant dams
E. All of the above
3. A positive marker gE bulk tank milk test result means:
A. The milking herd is vaccinated with marker gE vaccine
B. The milking herd contains at least 10-15 per cent of animals that have been exposed to BoHV-1
C. The herd has a high prevalence of marker vaccinated animals
D. The milking herd is not vaccinated
4. What would you advise a herd that has a positive gE bulk tank milk result?
A. Concentrate on bioexclusion
B. The herd is free from infection
C. Start full and regular vaccination combined with bioexclusion and regular monitoring
D. Start vaccination of the milking herd
Answers: 1D; 2E; 3B; 4C.





