Could Rapidly Mutating PCV2 Be Causing Health and Production Issues, Even in The Face of Vaccination?
Porcine Circovirus Type Two (PCV2) nearly decimated the pig industry in the late 1990s/early 2000s, but fortunately the creation of PCV2 vaccines was the silver bullet the industry desperately needed. Now nearly 100 per cent of commercial pig producers vaccinate their piglets against PCV2, and vaccination applied correctly does mostly prevent clinical disease, however despite a high vaccination rate, the PCV2 virus is still endemic globally. Dr Laura Hancox BVetMed PhD MRCVS, National Veterinary Manager, Zoetis UK Ltd, asks if the protection provided by vaccination is sufficient to prevent clinical PCV2 associated disease (PCV-AD), and is the fact that PCV2 can still be found replicating in our commercial pig herds relevant?
PCV – The Family of Viruses
PCV are tiny non-enveloped, single-stranded DNA (ssDNA) viruses. PCV are structurally very resilient with an ability to withstand both high temperatures and extremes of pH. This hardiness partly explains the endemic nature of the viruses, as eradication is practically impossible thanks to their stability within the environment.
There have been four genera of PCV identified to date:
PCV1: Discovered as an incidental cell culture contaminate in 1974. Non-pathogenic but endemic globally.
PCV2: Formally identified in 1991. Archived samples demonstrate presence from the 1960s. Endemic globally.
PCV3: First reported in 2016. Archived samples demonstrate presence from the 1990s. Clinically appears pathogenic, but has not yet formally met Koch’s Postulates. Reported in multiple countries around the world including the UK and Ireland.
PCV4: Isolated in China only to date. Reported as pathogenic, but not formally proven.
PCV2 is undoubtedly the most clinically relevant circovirus found in pigs to date, and PCV2 will be the focus of the article. However, it is worth noting that there are growing concerns over PCV3 as it has been associated with some noteworthy clinical disease around the world and currently there is no vaccine available.
PCV2 - Rapid Virus Evolution
PCV2 is the most rapidly changing ssDNA virus on the planet, partly due to its high mutation rate, and partly due to a high incidence of viral recombination:
• PCV2 - Rapid Mutation. Most DNA-based organisms have a ‘proof-reading’ mechanism to ensure any base substitutions/deletions/insertions are corrected as the DNA is being copied. Because PCV2 has such a tiny, circular ssDNA, there is no capacity for a proof-reading mechanism and hence any random errors which occur during virus replication are passed on to future virus copies. Mutations are most commonly seen in the Open Reading Frame 2 (ORF2) gene which encodes for the PCV2 protein coat. This ORF2 protein is the immunogenic part of the virus so any significant mutations could mean existing immunity from exposure or vaccination may not provide cross protection, e.g., vaccinal escape mutants.
• PCV2 – Frequent Virus Recombination. There can be multiple PCV2 strains on one farm and even within an individual pig. When a cell is infected with two PCV2 strains, the DNA polymerase can ‘jump’ from one DNA template to another, meaning two genomes can be spliced together to make a new strain. Around 33 per cent of PCV2 field strains are recombinant viruses.
The rapid rate of change of the virus has resulted in multiple genotypes, identified by a letter (PCV2a – PCV2h), with each genotype containing many sub-strains. Interestingly the last two formally identified genotypes, PCV2g and PCV2h, are thought to be a product of recombination events, demonstrating the relevance of viral recombination. PCV2a was the first genotype identified and was initially dominant globally, but then PCV2b became most frequently identified, followed by PCV2d (Figure 1). Interestingly PCV2d was initially called a mutant subtype of PCV2b as the two genotypes are genetically very similar (93–97.7 per cent), but it was subsequently placed in its own genotype.
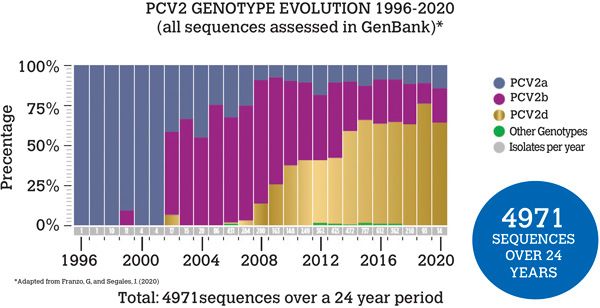
Figure 1: Global PCV2 genotypes
PCV2 Pathogenesis
PCV2 has a broad tropism and can apparently infect multiple tissues in pigs, which helps to explain why the virus can be involved in so many disease processes. The exact pathogenesis of PCV-AD is still not fully understood. This is partly due to a very mixed success in being able to recreate the disease syndromes experimentally. The experimental model which has been most successful at stimulating PCV-AD with PCV2 challenge has been a dual challenge model, e.g., challenge with another virus (Parvovirus or PRRS virus) simultaneously. This fact alone provides us with information of the immunomodulatory nature of the virus, as it seems another factor is needed to upregulate the immune system before clinical PCV-AD will occur.
The viral interaction with the immune system is also evident within some of the typical histopathology seen in PCV-AD such as lymphoid depletion in post-weaning multi-systemic wasting syndrome (PMWS). PMWS is probably the most noteworthy of the clinical syndromes associated with PCV2, particularly in unvaccinated herds, but this is closely followed by Porcine Dermatitis Nephropathy Syndrome (PDNS), and the virus is also recognised to have a role in respiratory disease, reproductive failure, enteritis and subclinical disease.
• Post-Weaning Multi-systemic Wasting Syndrome (PMWS)
PMWS may be diagnosed if the following criteria are met:
Increased mortality;
Clinical signs of wasting, pallor/icterus, commonly seen with coughing/dyspnoea or diarrhoea. Enlarged superficial lymph nodes. Clinical signs normally seen from around eight weeks of age (Figure 2);
Detection of PCV2 virus in lymphoid tissues with associated lymphoid depletion.

Figure 2. Courtesy: Dr Klaus Truschner
Porcine Dermatitis Nephropathy Syndrome (PDNS)
PDNS cases have irregular red/purple macules and papules on the skin and will be anorexic and depressed (Figure 3). They normally do not have a fever. They may be reluctant to move, and/or be stiff-gaited. PDNS may be diagnosed if the following criteria are met:
Presence of haemorrhagic and necrotising skin lesions, and/or swollen and pale kidneys with generalised cortical petechiae;
Presence of systemic necrotising vasculitis and necrotising, fibrinous glomerulonephritis;
Detection of PCV2 is not indicated as diagnostic for PDNS.
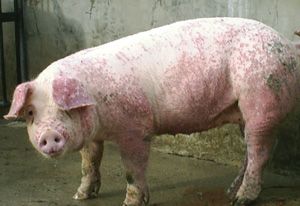
Figure 3. Courtesy: Dr Agnieszka Mańka
PCV2 – Subclinical Disease
Subclinical disease is the most common form of PCV2 infection. Subclinical PCV2 disease may be diagnosed in cases where there are no clear PCV-AD clinical signs, minimal or no gross or histopathological lesions, but with a level of PCV2 virus detectable in the pig associated with a decrease in average daily gain and an increased susceptibility to other infections. Subclinical cases of any disease can be difficult to identify, but this is especially true with PCV2 as it is an endemic virus and can commonly be found, even in vaccinated pigs.
So is the finding of the virus incidental, or could it be significant? The cost of subclinical disease is undoubtedly significant: Alarcon et al (2013) estimated the cost of PCV2 subclinical infection cases to be £82.30 per pig fatality and £8.10 per subclinical pig which survives until slaughter. Considering the scale of pig production, these figures could quickly multiply to eye-watering costs. So how can we diagnose subclinical PCV2 infection? A tricky question, and one to which there is no formal answer. However, below are some pointers which may aid diagnosis:
- Is growth seen to be suboptimal?
- Is there a higher than expected incidence of health issues?
- Can PCV2 be found actively circulating within the affected groups?
- Are there no specific clinical signs or lesions identified?
- Have other common diagnoses been disregarded?
If the answer to all of the above questions is yes, then it may be worth considering PCV2 subclinical disease in the herd.
PCV2 - Control
As discussed previously, eradication of PCV is practically impossible due to its stability in the environment, so management of the virus within the herd must be achieved; this is mostly done through vaccination. There are multiple PCV2 vaccines licensed in the EU. All vaccines launched before 2021 have been based on the ‘original’ PCV2 genotype PCV2a. Most of these licensed PCV2a-based vaccines have been able to demonstrate clinical cross protection against heterogenous genotypes, e.g., those that have since become more clinically relevant, PCV2b and PCV2d; however, these studies only demonstrated significant PCV2 immunity and clinical protection over unvaccinated controls.
A recent paper by Um et al was the first to compare clinical protection offered by a vaccine containing a single PCV2a genotype (Porcilis PCV M.hyo, MSD Ltd.) with a vaccine containing both PCV2a and PCV2b antigen (CircoMax Myco, Zoetis Ltd.); both vaccines also contained a Mycoplasma Hyopneumoniae antigen. The farm where the study was undertaken had been diagnosed with a subclinical PCV2 disease issue, and the genotype of PCV2 responsible had been identified as PCV2d. With relation to the PCV protection elicited within the study, it was shown that pigs vaccinated with the dual PCV2 genotype vaccine (CircoMax Myco, Zoetis Ltd.) compared to the PCV2a only vaccine (Porcilis PCV M.hyo, MSD Ltd.) had:
- significantly lower PCV2 viraemia levels at 10 weeks of age;
- significantly lower PCV2 faecal shedding at 10 weeks of age;
- significantly higher PCV2 ELISA titres at 10 and 16 weeks of age;
- significantly higher average daily weight gain (ADWG) from wean to finish.
Within the study, a technology by Epivax Ltd. was also used to assess epitope similarity between each vaccine and the PCV2d field strain that was identified as causing subclinical disease on the farm. Epivax analysis identified that epitope coverage between Porcilis PCV M.hyo and the field strain was 62.22 per cent, and the epitope coverage between CircoMax Myco and the field strain was 82.82 per cent. Although there are many aspects of a vaccine that elicit protective effects, it may be concluded that the higher degree of epitope coverage identified with the Epivax technology, resulted in the significant clinical improvements shown in the study with regard to the immune response, virus replication and ADWG in the CircoMax Myco vaccinated group. This study suggests that using vaccines with a broader PCV2 antigenic content, including PCV2b – which is identified more commonly than PCV2a and is closely related to the most currently clinically relevant genotype PCV2d – may be clinically beneficial.
Although it may be considered that updated PCV vaccines could provide a clinical benefit, PCV2a vaccines should still control PCV-AD in most scenarios. So if clinical PCV-AD is being seen where a vaccination regime is being applied, there are several areas which should be investigated:
- Is the vaccine cold chain being maintained correctly?
- Are all animals receiving their full dose and according to the product’s SPC?
- Are gilts getting a PCV booster before entering the breeding herd?
- Could PCV2 maternally derived antibodies be interfering with piglet vaccinal response?
- Have you tested for PCV3?
In summary, a good PCV vaccine, used properly, should prevent PCV clinical disease. However, it must be considered that these clinical disease scenarios may only be the tip of the iceberg. PCV2a-based vaccines undoubtedly saved the industry when first launched, but maybe it is time we re-evaluated their effectiveness, especially when considering the most common PCV2 genotypes currently circulating, and the increasing awareness of the impact of subclinical disease. As vets we should also be keenly aware of the ‘new kid on the block’, PCV3. With no vaccines available, the best approach is increasing awareness of units which may already be infected, and if it is believed a herd is negative, applying strict biosecurity in order to keep it that way!
References available on request.
1. How many Genera of PCV virus have been identified to date?
A. 3
B. 2
C. 4
D. 1
2. Mutations in the PCV virus are most commonly seen in which gene which encodes for the PCV2 protein coat?
A. Open Reading Frame 2 (ORF2) gene
B. Open Reading Frame 1 (ORF1 ) gene
C. Open Reading Frame 3 (ORF3) gene
D. E2 Ubiquitin Ligase
3. What percentage of PCV2 field strains are recombinant viruses?
A. 10%
B. 22%
C. 40%
D. 33%
4. Subclinical disease is the most common form of PCV2 infection. True or False?
A. True
B. False
5. Which genotype of PCV2 form the basis of the vaccines produced before 2021?
A. a
B. d
C. g
D. h
Answers: 1C; 2A; 3D; 4A; 5A.





