Providing adequate colostrum for foals and RVN’s role
Sinéad Farrell BSc VN, MSc, BSc (Hons) discusses some of the key issues around colostrum intake for foals
Introduction
Foals are born with no antibody protection and there is a complete lack of intrauterine transfer of immunoglobulins from the mare to the foal (Paradis, 2012). Foals are entirely dependent on passive transfer from colostrum. This is the process by which antibodies from the mare’s colostrum are absorbed into the bloodstream in the hours after birth to protect the foal against infectious disease (McCue, 2009). After the foal nurses, specialised cells which line the small intestine absorb the intact antibodies from the colostrum and transfer them to the foal’s blood (McCue, 2009). Absorption by these specialised cells is greatest in the first six to eight hours after birth and ceases by 24-36 hours of age (McCue, 2009). Most normal foals suckle from the mare within two to three hours of birth (Shepherd, 2010). The average 50kg foal needs to ingest 2-4L, or five to 12 per cent of their body weight, of good quality colostrum within the first few hours of life to acquire sufficient antibodies (McCue, 2014: Bedenice 2020). If the foal does not receive and absorb sufficient colostral antibodies, failure of passive transfer (FPT) occurs (Shepherd, 2010), resulting in increased neonatal mortality and morbidity (Drogoul et al., 2008).
Colostrum: what is it?
Colostrum is a yellow, sticky and thick fluid which is produced by the mare’s mammary glands during the last few weeks of pregnancy (McCue, 2009, 2014). It is made up of water, proteins, fats, carbohydrates and electrolytes (McCue, 2014). It is higher in gross energy, total protein, specific gravity and Vitamins A and E than an equal amount of milk (Paradis, 2012). Another advantage of ingesting colostrum is that it initiates a laxative effect in the foal, facilitating defaecation of meconium (Vaala, 2007).
Colostrum is a rich source of antibodies which are vital for immune function in the newborn foal (McCue, 2014). There are many immunoglobins which are present in colostrum but the major ones are IgG(T), IgGa and IgGb; other immunoglobins present in smaller amounts include IgM and IgA (Vaala, 2007). At parturition, the IgG level of normal colostrum should be >3000mg/dl. It can exceed 9000mg/dl and the concentration is mare-dependent (Vaala, 2007).
Poor quality colostrum can be caused by a variety of factors which include: poor maternal immune or nutritional status, premature lactation, placentitis, premature placental separation, advanced maternal age, maiden pregnancies, breed and ingestion of endophyte-contaminated fescue grass or hay (Vaala, 2007; Veterian Key, 2016).
Colostrum assessment
Good quality colostrum is thick, sticky in texture and yellow in colour whereas poor quality colostrum can be watery and is white in colour (McCue, 2014). The colour of colostrum is not necessarily indicative of its quality (Chavatte et al., 1998). Colostrum quality can be assessed using a variety of methods both visually and quantitatively (Shepherd, 2010). Optical density and specific gravity are both consistent with the content of antibodies in the colostrum (McCue, 2014). The use of a digital or handheld Brix refractometer is one of the easiest yet most useful ways of determining immunoglobin G (IgG) levels (Shepherd, 2010; McCue, 2014).
Refractometry measures the concentration of dissolved solids in a solution (McCue, 2014). A small amount of colostrum is placed on to the prism on the Brix refractometer and the light plate is closed and the colostrum is evenly spread across the prism (McCue, 2014). The refractometer is then turned towards a light source and the refraction of the light through the colostrum is evaluated as a percentage (McCue, 2014).
Colostrum that has a high concentration of dissolved solids (high levels of antibodies) will have a large degree of scattered light and will subsequently have a high Brix percentage score (Table 1). Whereas colostrum with low amounts of dissolved solids (low antibodies) will have a lower degree of light scatter and will have a lower Brix percentage score (McCue, 2014). The use of the Brix refractometer to evaluate equine colostrum quality has been shown to be highly correlated with the level of plasma antibody levels in the foal (McCue, 2014).
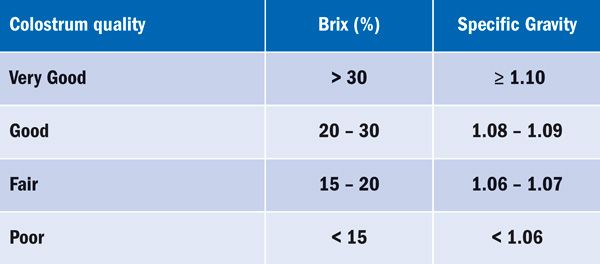
Table 1: Brix scores in percentage and specific gravity measurements of the different categories of colostrum (McCue, 2014).
Digital refractometry can also be used. These are pocket-sized handheld devices which are extremely easy to use (Figure 1). They utilise the same principles as the Brix refractometer: a drop of colostrum is dropped onto the prism, the lid is closed over and the quality of the colostrum is read by the refractometer and displayed as a percentage on the screen within a few seconds (Kruuse, n.d.). This is a reliable and easy to use machine, though it can be quite expensive to buy outright at the beginning.

Figure 1: Digital refractometer.
Another type of digital refractometer which is available is the ‘dip style’ digital refractometer. This works by dipping the silver tip of the refractometer into the colostrum and stirring it, press the start button and the results will appear in the Brix scale on the digital screen within three seconds (Golden Calf company, n.d.).
An alternative way of measuring the specific gravity of colostrum is by using a colostrometer. An exact volume (15mls) of colostrum is added to a specially-designed glass chamber which is calibrated. The colostrum-filled chamber is then floated in distilled water in a graduated cylinder (McCue, 2014). The colostrum’s specific gravity is measured by reading the scale on the glass cylinder at the water line. Colostrum with low antibody levels has a lower density and lower specific gravity, and colostrum with high antibody levels has a higher density and high antibody levels. This method can be inaccurate as a precise volume of colostrum must be used, otherwise it can lead to significant errors in the determination of the specific gravity (McCue, 2014). A colostrometer is not very convenient to use in field conditions, as it requires a much larger volume of colostrum to be collected from the mare, and measurements may not be repeatable (Chavatte et al., 1998). It is also easily broken.
Calibration of the Brix and digital refractometers should become routine to ensure accurate results are being obtained. Distilled water or a manufacturer’s recommended calibration solution should be used and these solutions should produce a reading of zero (Bovine Veterinarian, 2017).
Brix refractometer:
The calibration solution/distilled water should be wiped off the prism of the refractometer before testing colostrum (Bovine Veterinarian, 2017). A few drops of well-mixed colostrum should be placed on the prism and the cover closed. The refractometer should be held up to the eye and pointed towards a light source. The result should be read from where the light and dark interfaces meet.
Digital refractometer:
The refractometer should be calibrated before use and the prism wiped clean before adding the sample (Kern optics, 2015). The read/measure button should be pressed and a reading will appear on the screen (Kern Optics, 2015).
Colostrum absorption
Absorption of colostrum is achieved by a process called pinocytosis, which involves specialised epithelial cells within the small intestine (Ayala and Oliver-Espinosa, 2016; Bernard and Barr, 2012; Sangild, 2003). These specialised cells engulf large molecules, such as immunoglobulin droplets from the intestinal lumen, and transfer these droplets in small vacuoles for transport across the cytoplasm, from where the contents are discharged into the lymphatic vessels (McCue, 2014; Bernard and Barr, 2012). The antibody molecules are then transported through the lymphatic system and enter the bloodstream intact (McCue, 2014). These specialised pinocytotic cells have a short life span and maximal absorptive capacity is reached at about 12-16 hours of age, followed by a steady decline up until 24 hours of age (Bernard and Barr, 2012). The pinocytotic cells are then replaced by mature epithelial cells which are not capable of absorbing large macromolecules (Bernard and Barr, 2012). The replacement of these cells is not dependent on the ingestion of colostrum and will occur whether or not colostrum has been ingested (Bernard and Barr, 2012).
It is also important to mention that not just colostrum is absorbed across these specialised epithelial cells. Bacteria can also be absorbed across the intestine and, therefore, it is recommended to ensure the foaling box, as well as the hindlimbs and teats of the mare, are clean. The udder should be washed with mild soap and warm water to remove bacteria and dirt (McCue, 2014). Detergents containing chlorhexidine should be avoided as they can leave an aftertaste which can deter the foal from suckling. Udder cleaning can be done after the foal is born but before it suckles.
Has the foal received enough colostrum?
Healthy foals that have consumed sufficient good quality colostrum should have a serum IgG concentration of
>800mg/dl by 12-24 hours of age (Vaala, 2007). A foal with FPT has a serum IgG concentration of <400mg/dl at 24 hours of age (Vaala, 2007). Partial FPT is defined as a foal with a serum concentration of between 400 and 800mg/dl (Vaala, 2007).
There are several tests available to measure plasma IgG levels and each has its advantages and disadvantages.
Single Radial Immunodiffusion (SRID) testing is considered the gold standard and is accurate but expensive and takes 18-24 hours to get results, so is impractical in most cases (Vaala, 2007; Veterian Key, 2016). This test uses antiserum to equine IgG and compares patterns of precipitation produced in agar gel to known standards (Bernard and Barr 2012). This test is both quantitative and specific for IgG.
Turbidity tests to interpret immunoglobulin content have been developed and are quick and inexpensive. The most common used is zinc sulfate (Bernard and Barr 2012). Zinc sulfate is added to foal serum which causes the globulins to become insoluble resulting in a cloudy appearance. The optical density is then measured spectrophotometrically (Bernard and Barr 2012).
Latex agglutination determines immunoglobulin content (Bernard and Barr 2012). The latex agglutination test uses latex particles coated with an anti-equine IgG, the particles agglutinate in the presence of Immunoglobulins (Bernard and Barr 2012). The test takes ten minutes to obtain a result, can be used on either whole blood or foal serum and is reliable (Bernard and Barr 2012).
ELISA tests, which are an enzyme linked immunosorbent assay, can also be used (Bernard and Barr 2012). These assays use a membrane filter to measure immunoglobulins in foal serum or whole blood and are semi-quantitative (Bernard and Barr 2012). An example of such a test is the Idexx SNAP Foal IgG test (Figure 2) (Idexx, 2020). There are colour calibration spots which are compared to the intensity of the colour reaction on the test filter (Bernard and Barr 2012). It is a quick, easy and accurate test to use and takes less than 10 minutes to obtain a result.
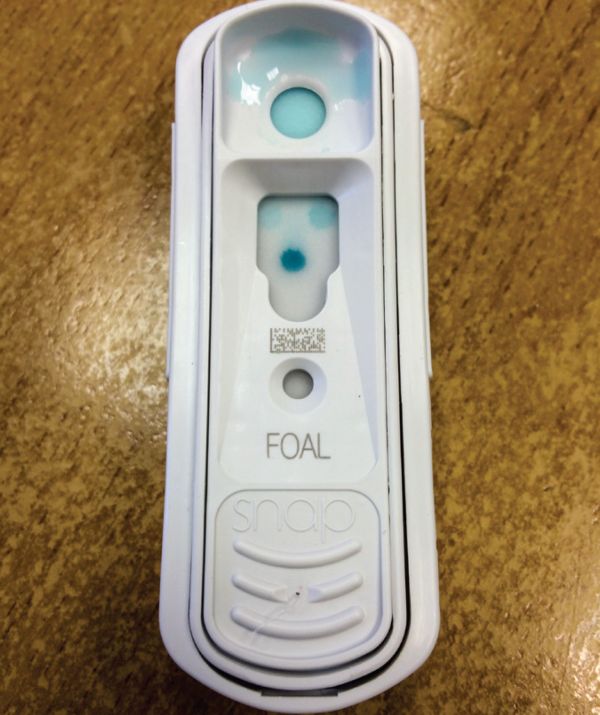
Figure 2: Idexx SNAP foal IgG test with a reading of >800mg/dl.
Foals at 12 hours of age:
A blood sample can be collected from the newborn foal at approximately 12 hours of age and after it has ingested colostrum to evaluate the IgG levels before the closure of gastrointestinal tract to antibody absorption (McCue, 2009). If the IgG levels are less than 400mg/dl at this stage, oral supplementation with frozen-thawed colostrum or a commercial colostrum substitute should be given (McCue, 2009). Foals with a serum concentration of between
400-800mg/dl may need intervention and therapy is dependent on the medical condition of the foal and the potential for pathogen exposure (McCue, 2009). Foals with partial failure of passive transfer are at risk of developing infections and can benefit from receiving extra IgG supplementation. However, if they are born into a clean environment with good preventative management, supplemental IgG may not be required (McCue, 2009).
Foals at 24 hours of age:
Testing at this age will establish the full extent of immunoglobin absorption (McCue, 2009). No further antibodies will be absorbed if oral supplementation with colostrum is given after 24 hours of age (McCue, 2009). Foals older than 24 hours that have been identified with FPT require intravenous administration of hyperimmune plasma or a commercial IgG preparation to increase blood antibody levels successfully (McCue, 2009). It is easier, cheaper, safer and more effective to test early and provide oral administration of these antibodies, rather than administering plasma transfusions (McCue, 2009).
Why might failure of passive transfer occur?
There are many possible causes for failure of passive transfer and these include:
- Inadequate production of immunoglobulin-rich colostrum due to premature lactation, premature delivery, agalactica, and failure to produce high quality colostrum (Bernard and Barr, 2012).
- Inadequate colostrum being administered to the newborn foal due to any factor that prevents the foal suckling from the mare, complete or partial rejection of the foal (Bernard and Barr, 2012).
- Inadequate absorption due to limited sites in the intestine for pinocytosis, inadequate immunoglobulin transfer to systemic circulation, early closure of the gastrointestinal tract and using calories by catabolism (Bernard and Barr, 2012).
Foals may develop FPT even though they have ingested sufficient quantities of good quality colostrum within the correct timeframe of six to eight hours; this is due to a failure of the specialised cells to absorb ingested immunoglobulins (Veterian Key, 2016). This is most common in dysmature or premature foals due to immature gastrointestinal function, but it may also occur in full term foals (Veterian Key, 2016). Glucocorticoids cause the epithelial cells in the intestine to mature quickly and they lose their capacity to absorb, if these are released secondary to stress during parturition it can cause immunoglobulin absorption to be impaired (Veterian Key, 2016).
Alternative sources of colostrum
A colostrum bank (Figure 3) or storage on site is the best management method to implement during foaling season, as it is not known in advance whether individual mares will produce sufficient quality colostrum for their foal. Optimal colostrum donors are mares who have had one or more foals in the past and are between four to 15 years of age (McCue, 2014). Colostrum should be tested before banking to ensure quality, and only good or very good colostrum should be kept for future use. Blood samples from potential donor mares should be tested for the presence of anti-RBC antibodies and only mares which test negative should have their colostrum banked to decrease the risk of neonatal isoerythrolysis (McCue, 2014).

Figure 3: Colostrum bank, 250ml frozen colostrum IgG level >800mg/dl.
Collecting colostrum is relatively simple; the udder of the mare should be washed with soap and warm water to remove bacteria and dirt. Colostrum is recommended to be collected from one side of the mammary gland in the first hour after foaling and before the mare’s own foal has suckled (McCue, 2014). A total of 240-480ml can be safely collected from the mare without a detrimental effect on her foal’s intake (McCue, 2014).
Collection of colostrum can be achieved using an inverted syringe, hand milking the mare or by using a commercial milking device. The inverted syringe can be made relatively easily and is a cheap option (McCue, 2019; Shepherd, 2010). The end is cut off a 60ml syringe, the plunger is reversed and placed into the end which was cut off, and the flared end of the syringe is placed over the mare’s teat against the udder (Figure 4) (McCue, 2019). By gently pulling on the plunger of the syringe, suction will be created and colostrum drawn into the barrel (McCue, 2019). This can be repeated until the required volume has been collected.
Colostrum which has been harvested should be poured through cheesecloth or gauze into a clean storage container (such as a plastic container or heavy, sealable plastic bag) which is labelled with the donor mare’s name, collection date and IgG concentration of the colostrum (McCue, 2019). Colostrum can be safely frozen for up to two years at -20 degrees Celsius (McCue, 2014). Frozen colostrum should be thawed gently for use. Do not microwave it, place it in a hot water bath or near a heat source, as temperatures of >40°C will destroy the antibodies making it unusable (McCue, 2019). The colostrum should be thawed gradually in room temperature water and should be administered immediately (McCue, 2019). Plastic bags of colostrum which are frozen while flat will thaw more rapidly due to their greater surface area. These bags of colostrum can be stored upright in the freezer wrapped in bubble wrap or in a box of wood wool to ensure the plastic doesn’t get damaged and the corners of the bag are protected in the freezer.
Colostrum being fed to the foal can be administered by orogastric tube or by bottle feeding. This decision depends on how weak the foal is and whether there is a strong suckle reflex present. If there is an absent or weak suckle reflex, bottle feeding should not be used as the risk of colostrum aspiration is high. Overall, bottle feeding has a greater risk of aspiration pneumonia occurring than orogastric intubation (Paradis, 2012).
Frequent cleaning and disinfection of the items being used to feed the colostrum are required to prevent microorganisms being absorbed across the gastrointestinal tract (Lien, 2015). All feeding utensils should be washed thoroughly with warm soapy water, rinsed and then placed in a large, clean plastic container with a lid. The container should be filled with 5L of cold water and 30ml of Milton sterilising fluid; this volume can be adjusted to the size of the container (Milton, 2020). Ensure all items are fully submerged and no air bubbles are trapped within tubes or bottles. These items can stay in the solution until needed and rinsing is not required before use. This solution should be changed and replaced every 24 hours (Milton, 2020).
Fresh bovine colostrum, or bovine-derived colostrum replacer, has been shown to be absorbed by the small intestine and provides some passive immunity to foals (Madigan, 2014). However, it does not provide the specific antibodies required to protect the foal against all equine pathogens (Madigan, 2014). It is recommended that its use be restricted to situations where there is no access to equine colostrum (Madigan, 2014).
Commercial serum concentrate products for oral use are available, along with Lyophilised Equine IgG which is derived from equine serum and is absorbed by the small intestine if administered in the first 12 hours of life (Madigan, 2012). Hyperimmune equine plasma can also be administered intravenously in foals which have failed to receive any or little colostrum by 24 hours of age.
Plasma can also be collected from a healthy donor; if compatibility is a concern, the dam’s plasma can be used. This can be a cost-effective way to treat FPT. Healthy foals with partial FPT should receive one litre of plasma. However, foals with total FPT may require two or more transfusions over several days to boost plasma IgG levels. IgG levels should be measured four to 12 hours post-transfusion to get the most accurate result (McCoy, 2014).
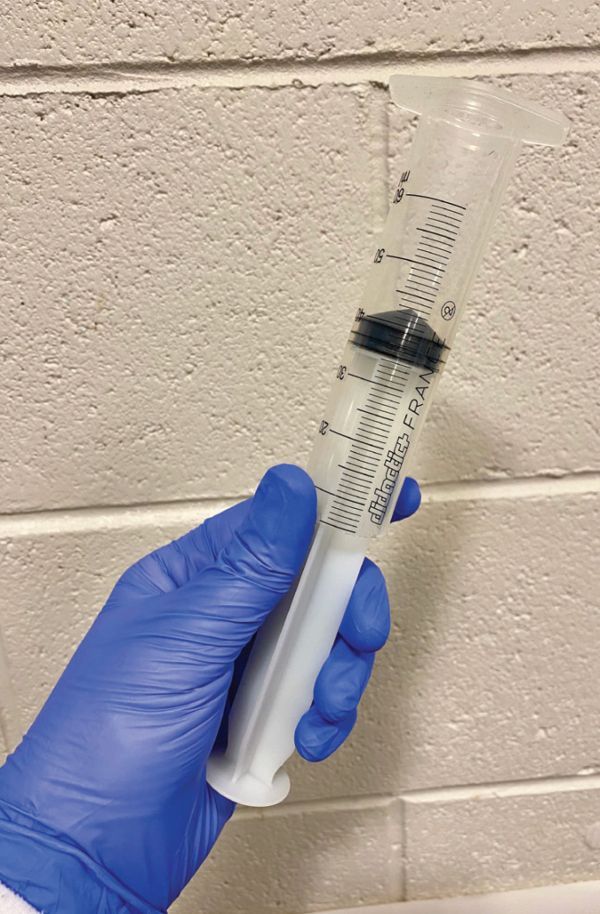
Figure 4: Inverted 60ml syringe used to milk the mare.
Conclusion
It is extremely important for a foal to receive sufficient colostrum in the 24 hours following birth. This article discusses the ways you can test colostrum to determine its quality and to test the foal to determine if the foal has had enough colostrum. It also discusses what to do if the foal hasn’t had enough colostrum and ways to prevent failure of passive transfer. The digital and Brix refractometers and Idexx snap tests should form part of the foaling kit, and the veterinary nurse can educate clients in their use. RVNs may assist breeders in the prevention and management of FPT, thereby ensuring foals obtain the best possible start in life.
-
Ayala, M. S. and Oliver-Espinosa, O. J. (2016). Risk Factors Associated With Failure of Passive Transfer of Colostral Immunoglobulins in Neonatal Paso Fino Foals. Journal of Equine Veterinary Science, 44, pp. 100–104.
-
Bedenice, D. (2020). Failure of passive transfer [online]. Available from: https://www.merckvetmanual.com/management-and-nutrition/management-of-the-neonate/failure-of-passive-transfer [accessed 31 January 2020].
-
Bernard, W. V. and Barr, B. S. (2012). Equine Pediatric Medicine. Ed. London: Manson Publishing.
-
Bovine Veterinarian. (2017). Five steps to using a refractometer. Available from: https://www.bovinevetonline.com/article/five-steps-using-refractometer [accessed 30/06/2020].
-
Chavatte, P., Clement, F., Cash, R and Gronget, J-F. (1998). Field Determination of Colostrum Quality by Using a Novel, Practical Method. Proceedings of the Annual Convention of the AAEP, 44, pp. 206–209.
-
Drogoul, C., Clemont, F., Ventorp, M., Curadi, M.C. and Orlandi, M. (2008). Equine passive immune transfer trough colostrum. Proceedings of the 4th European Equine Nutrition and Health Congress, pp. 23–27.
-
Golden calf company. (n.d). Calf Lab Testing [online]. Available from: http://www.goldencalfcompany.com/calf-lab-testing/colostrum-testing/ [accessed 12 April 2020].
-
Kern optics. (2015). Operating instructions Digital refractometer. Available from: https://docs.rs-online.com/08c5/0900766b814b98c9.pdf [accessed 30/06/2020].
-
Kruuse. (n.d). Colostrum Digital refractometer [online]. Available from: https://kruuse.com/products/3-instruments/3-5-examination/3-5-6-single-parameter/3-5-6-3-refractometers/colostrum-digital-refractometer [accessed 12 April 2020].
-
Lien, L. (2014). Neonatology. In: Lien, L., Loly, S. and Ferguson, S.. eds. Large animal medicine for veterinary technicians. Iowa: Wiley Blackwell, pp.145-177.
-
Madigan, J. E. (2014). The Manual of Equine Neonatal Medicine, Ithaca NY (www.ivis.org).
-
McCoy, A.M., McKenzie, E. and Loly, S. (2014). Clinical Procedures. In: Lien, L., Loly, S. and Ferguson, S.. eds. Large animal medicine for veterinary technicians. Iowa: Wiley Blackwell, pp.145-177.
-
McCue, P. M. (2009). Failure of Passive Transfer: Early Testing is the Key. Colorado State University - Equine Reproduction Laboratory.
-
McCue, P. M. (2014). Equine Colostrum: The Elixir of Life for a Newborn Foal. Colorado State University - Equine Reproduction Laboratory.
-
McCue, P. M. (2019). Clinical Equine Reproduction Volume 2 [online]. Colorado. Available from: https://books.apple.com/us/book/clinical-equine-reproduction-volume-2/id1458034274 [accessed 15 April 2020].
-
Milton. (2020). Sterlising Fluid. Available from: https://www.milton-tm.com/en/consumer/products/sterilising-fluid [accessed 16/07/2020].
-
Paradis, M. R. (2012). Normal foal nutrition. 58th Annual Convention of the American Association of Equine Practitioners, 58, pp. 132–137.
-
Sangild, P. T. (2003). Uptake of colostral immunoglobulins by the compromised newborn farm animal. Acta Veterinaria Scandinavica, Supplement, 98, pp. 105–122.
-
Shepherd, C. (2010). Post-parturition examination of the newborn foal and mare. In Practice, 32(3), pp. 97–101.
-
Vaala, W. (2007). New perspectives on the late-term mare and newborn foal. AAEP Proceedings, 53, pp. 281–292.
1. How much good quality colostrum should a 50kg foal ingest in the first few hours of life?
A. 3-5 litres
B. 10 litres
C. 2-4 litres
2. Apart from passive transfer, what is an additional benefit of ingesting colostrum?
A. Induces a laxative effect allowing meconium to be defaecated.
B. It helps the foal to urinate appropriately.
C. It helps the mare and foal bond better.
3. What does good quality colostrum look like?
A. Thick, watery in texture, white in colour
B. Thick, sticky in texture, yellow in colour
C. Thin, sticky in texture, yellow in colour
4. What percentage reading does good quality colostrum have on a Brix refractometer?
A. < 15 %
B. < 30 %
C. 15-20 %
D. 20-30 %
5. ELISA tests such as Idexx SNAP foal IgG tests are the most convenient, easy to use and accurate test available for checking for FPT in foals.
A. True
B. False
6. Failure of passive transfer can still occur even if the foal has ingested sufficient amounts of good quality colostrum within the appropriate time frame.
A. False
B. True
Answers: 1C; 2A; 3B; 4D; 5A; 6B.





