Pressing the ‘reset’ button after Covid-19
Niamh McGill MVB looks at how human influences are affecting the world on a large and damaging scale, and specifically explores the impact of emerging infectious diseases
We live in a world dominated by human influences, an age now popularly known as the ‘Anthropocene’. Human influences now effect most processes on earth and there is no denying that human activity and profound economic growth have driven significant climate change and biodiversity loss as we rapidly exceed the planetary boundaries. We have engaged in the large-scale conversion of natural habitats to agricultural crops and urban areas while we have simultaneously degraded the integrity of unconverted natural systems through activities like natural resource extraction, logging and farming. There have been many recent attempts to map the level of anthropogenic environmental modification across the land and ocean with some estimates showing that almost 80% of both realms have clear evidence of human interference. There are many who would claim we are acting as Gods with pronouncements that it is us rather than nature ruling the world but we know this to be but a fragile and temporary position if recent events are to teach us anything.
The Anthropocene has been irrevocably rocked by a coronavirus which has successfully adapted from its evolutionary bat origin and we have been rendered helpless as it has acquired the traits required to relentlessly spread across the globe’s eight billion susceptible humans. Unfortunately, nothing can drive a message home better than a widespread health crisis and devastating economic impacts and this virus has, in no uncertain terms, exposed the fragility of an accumulation-based, capitalist society which ignores ecosystem health and the implications of global change. At the time of writing (early September) the world had surpassed 28 million confirmed cases of severe acute respiratory syndrome coronavirus 2 or SARS-CoV-2 (Covid-19) with almost one million deaths according to Johns Hopkins University (Figure 1). Quarantines and widespread travel restrictions have become the norm as countries grapple to get a grip on the mounting death toll and economic cost of the Covid-19 crisis while two central questions are being asked repeatedly: where did this virus come from and how can we prevent future pandemics like this from happening again?
Emerging infectious diseases and Covid-19
Emerging infectious diseases (EIDs) are variably defined as ‘infections that have newly appeared in a population or have existed previously but are rapidly increasing in incidence or geographic range’. EIDs not only describe the spread of newly evolved pathogens but also take into consideration pathogens that are spreading to new geographic ranges or populations (eg. lyme borreliosis), pathogens appearing in areas undergoing ecological transformation that may have a significant impact on human or animal health (eg. chytridiomycosis) and pathogens that may be reappearing (or re-emerging) after a decline in incidence (eg. measles). EIDs are dominated by zoonoses, approximately 75% of which have their origins in wildlife and their incidence has dramatically increased over the last two decades (Figure 2), largely driven by widespread ecosystem degradation as well as both the legal and illegal wildlife trade.
Ecosystem perturbations are now widespread across the globe because of climatological variation, landscape alterations, anthropogenic factors and globalisation. The resultant ecosystem degradation has increased the incidence of EIDs originating from wildlife and altered the epidemiological patterns of many diseases such that we can clearly recognise that ecological health and the epidemiological features of agent and host interactions are undeniably linked. There are multiple examples in the literature which support the linkage between ecological degradation and the increased risk of zoonotic disease outbreaks originating from wildlife. Australian scientists have shown that declining eucalyptus habitat altered flying fox foraging behaviour and increased spillover risk of Hendra virus to humans while the emergence of Nipah virus in 1998 was linked to the ecology of bats in changing landscapes. Deforestation, forest degradation, fragmentation, habitat encroachment and expansion of human infrastructure are just some examples of the land-use changes that can perpetuate the introduction of pathogens into new species populations while the legal and illegal wildlife trade has connected biodiverse forest areas to markets and susceptible human populations.

Figure 1: The Johns Hopkins University Covid-19 dashboard on September 10, 2020.
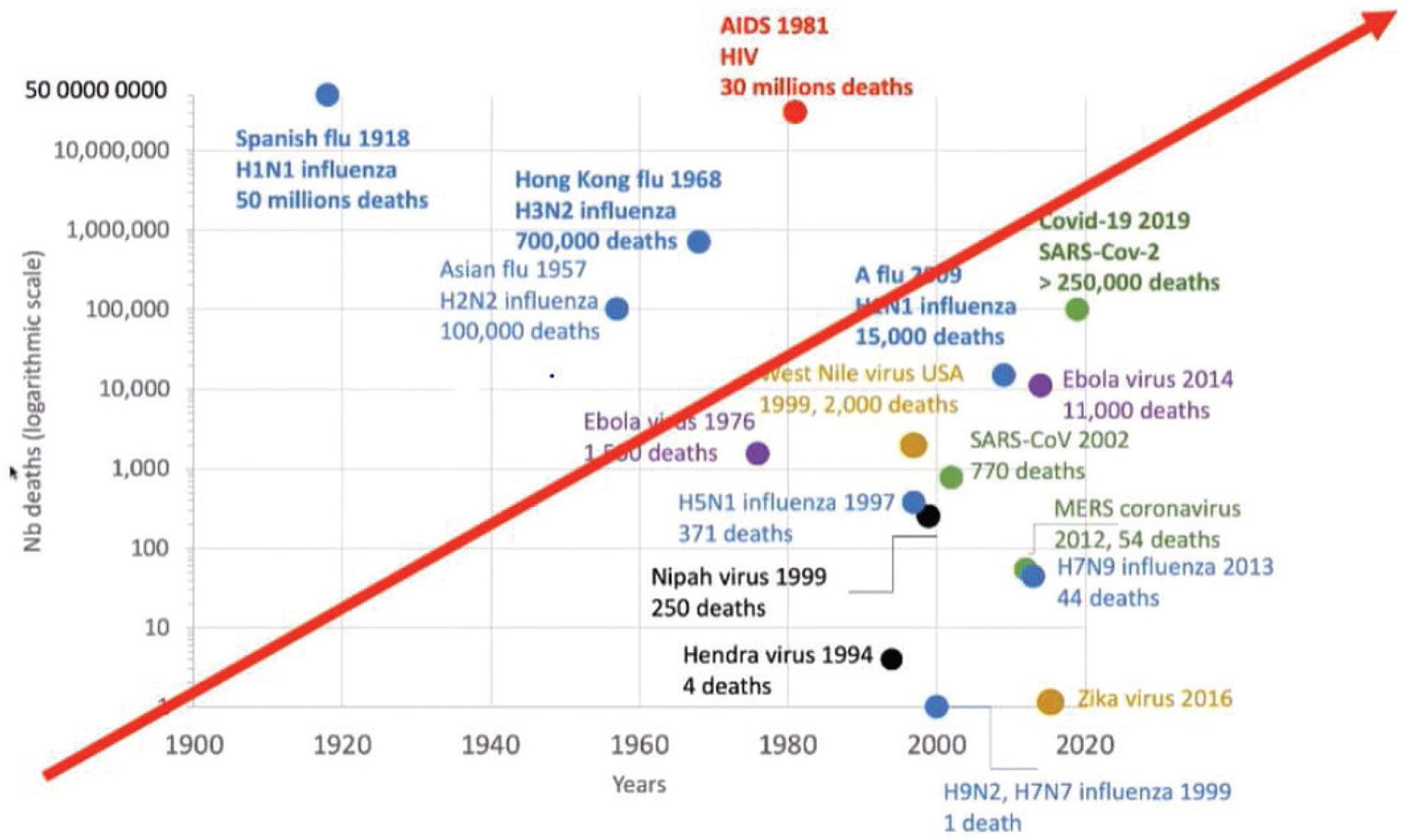
Figure 2: Emerging viral diseases (Lowenthal et al, 2015).
EIDs affecting livestock
While disease outbreaks in livestock are not a new phenomenon, modern trends have significantly increased the risks associated with them. The huge demand for livestock products around the world has led to the emergence of increasingly complex livestock systems and value chains in which the animals are selected based on production traits rather than disease resistance. This coupled with the enhanced biosecurity measures on farms has also contributed to the increased vulnerability of livestock to disease threats, as programmes aimed at the national eradication of infectious diseases mean that animals are immunologically naïve and, thus, at increased risk from pathogen incursions. With increased transportation of livestock and livestock products internationally for trade, intensified farming practices, expanding vector ranges as a result of climate change and the evolution and mutation of pathogens themselves, the likelihood of future serious epidemics in livestock populations as a result of emerging and remerging infectious diseases is growing. The principles of stringent biosecurity and surveillance for emerging disease threats are nothing new to the large-animal practitioner as robust systems of surveillance, prevention and control of animal diseases, including the responsible use of veterinary medicines, can serve to minimise risks to both animal and human health.
Impacts of EIDs
EIDs that affect both humans and animals have an impact on human society, animal production and welfare and conservation of biodiversity. They present unique challenges in terms of public health protection and economic stability, and the impacts of EIDs on both human and animal health over the last 20 years cannot be overstated as illustrated by the following examples:
The impact of the 2002-2004 SARS coronavirus epidemic (774 deaths) on tourism, food and travel in mainland China alone was estimated at US$8.5 billion (Beutels et al, 2009). The total global cost, as a result of lost economic activity, is estimated to have been around $40 billion (Knobler et al, 2004);
The 2014 Ebola outbreak in West Africa cost an estimated US$2.2 billion in GDP alone, killed over 11,000 people and wiped out many of the development gains in Guinea, Liberia and Sierra Leone (CDC, 2016); and
The unfolding Covid-19 pandemic will have an immense cost to the world. Recent estimates anticipate monetary costs will reach US$1-2 trillion with huge additional costs to human life and wellbeing as well as economic and political systems.
Impacts of EIDs on livestock:
- Bovine spongiform encephalopathy (BSE) resulted in losses to the UK economy of £607 million annually (Pretty et al, 2000) at the height of the disease outbreak in 1997;
- The 2001 foot and mouth disease outbreak in the UK led to the slaughter of 2,382,000 sheep and cattle (Defra, 2010);
- Schmallenberg virus (SBV) emerged among domestic and wild ruminants in Europe in 2011 and had a significant economic impact owing to significant trade restrictions and an overall drop of 11-26% in the number of semen doses exported outside Europe (EFSA, 2012).
- Bluetongue is an economically important transboundary disease that is listed by the World Organisation for Animal Health (OIE) reflecting the ability of this vector-borne viral disease to cause large economic losses due to high mortality rates, reduced productivity, trade restrictions and surveillance and control strategies. The expansion of culicoides-transmitted arboviral diseases (Bluetongue, Schmallenberg) across every continent highlights the potential of these diseases to threaten naïve ruminant communities and have substantial economic consequences. An epizootic of highly virulent BTV-8 began in Western Europe in 2006 with country-specific costs associated with production loss, trade restriction and control measures were estimated to be as high as €207 million.
- Highly Pathogenic Avian Influenza (HPAI) is a notifiable, potentially zoonotic disease that presents a constant threat to the poultry industry as a result of wild and migratory birds. Monetary estimates of global HPAI losses from the outbreaks since 2003 run into billions of US dollars due to widespread depopulations, trade restrictions and stringent biosecurity and control measures.
‘Spillover’ and the occurrence of Covid-19
Bats (order Chiroptera) are known to be reservoirs of a large number of zoonotic viruses that have caused multiple disease outbreaks in human and livestock populations in the past. A large diversity of coronaviruses, including SARS-related coronavirus and Middle-eastern Respiratory virus (MERS), have been discovered in bats and studies of these suggest a high capacity for transmission across the species barriers through close contact or consumption. Concerns of a serious ‘spillover’ event have been voiced for several years with a University in Hong Kong concluding as far back as 2007 that “the presence of a large reservoir of SARS corona-like viruses in horseshoe bats coupled with the culture of exotic mammal consumption in China was a ticking time bomb.” Recombination is widely accepted to play a central role in the evolution of coronaviruses and viral sequencing in the SARS and MERS outbreaks demonstrated that they originated from zoonotic transmission with intermediate hosts between the bat reservoirs and humans – a common pathway leading to coronavirus zoonosis. Although there are still no definitive answers as to the exact origins of Covid-19, specialists in the field of virology and immunology have suggested that the virus occurred naturally, likely through a recombination event among coronaviruses in an area where humans and wildlife were in direct contact for prolonged periods of time. There is significant evidence to suggest that the live animal markets in Wuhan, China were the original source of virus transmission, although the precise location, time and mechanism of the spillover event is still unknown and likely will be for several years. Bats at these markets are routinely transported and stored in very close contact with large groups of illicitly traded wild animals that are eventually consumed by humans. This form of transportation and storage creates an interface between different wild animals and humans which has aptly been described as ‘a cauldron of contagion’, providing a unique opportunity for the transmission and spread of pathogens between several species that would not ordinarily interact in the wild (Figure 4).
In general, these interfaces may be locations of increased human-wildlife contact such as forest buffer zones and wildlife markets, as well as locations with substantial land-use change such as logging areas. EID transmission at dynamic wildlife-livestock-human interfaces occurs via aerosols, water, shared food, bodily secretions and vector bites and live markets are considered the perfect petri dish for the creation of a novel virus.
It is hypothesised that Covid-19, a betacoronavirus similar to SARS and MERS, originally transmitted from bats to another susceptible intermediary host before being transmitted to humans after a recombination event. Many intermediary hosts have been implicated at this point including pangolins, racoon dogs and porcupines, although it will likely be several years before any definitive conclusions on the origin of the outbreak can be drawn.
But we must remember that zoonotic pandemics are not a new phenomenon and we have had very clear and loud warning that such a pandemic was on the horizon. Unfortunately, however, none of this was picked up by policy makers or administrations and we have been found sorely unprepared in the face of the biggest global health crises of this century. So, what can we do to reduce the chances of such a pandemic from happening again in the near future?

Figure 3: Live animal markets in Southeast Asia with illicitly traded wild animals.

Figure 4: A human-wildlife interface around a temple in Sariska Tiger Reserve, India. Image shows a monkey urinating over a human water source.
Prevention
The Covid-19 pandemic has resulted in a surge in public interest in the risk factors that facilitated such events and commercial wildlife markets and wildlife trade have been the subject of much scrutiny. On April 15, Chris Walzer, head of the Wildlife Conservation Society’s wildlife health programme, called for an immediate and permanent ban on the commercial trade of wildlife for human consumption, at a briefing of the US Congress’s International Conservation Caucus. Dr Anthony Fauci, director of the National Institute of Allergies and Infectious Diseases, also insisted on a global closure of wet markets insisting that the current crisis was a ‘direct result’ of this practice. While the control of wet markets and global law enforcement to address wildlife trafficking represents a high priority in targeting high-risk conduits for the transfer of disease, what should now be clear is that this pandemic is not only about bat soup, civets or pangolins, it’s about how we interact with our natural environment and the world around us. Given that there are estimated to be over 1.7 million unknown viruses currently in circulation, to address this problem using a virus species approach makes no sense whatsoever from an epidemiological point of view. Rather than focusing on the virus, we need to shift our focus towards our interactions with the natural world – as discussed above, there are numerous clear lines of evidence supporting the conclusion that declines in the integrity of ecosystems increase the global risk of zoonotic disease spillovers and, thus, the maintenance of healthy ecosystems needs to become a global priority.
Opportunities
The costs for nature are exploding and we are now presented with the opportunity to revisit dysfunctional paradigms and break away from the silo mentality that has fragmented our collective thinking for too long.
This pandemic has laid bare many opportunities for change and drives home the importance of not viewing ourselves as separate and distinct from nature. The simple truth that ‘health connects all species’ – an idea shared by many collaborative and transdisciplinary approaches such as One Health, Global Health, Planetary Health and Conservation Medicine (Figure 5).
Figure 5: Four core components of an effective disease surveillance programme.
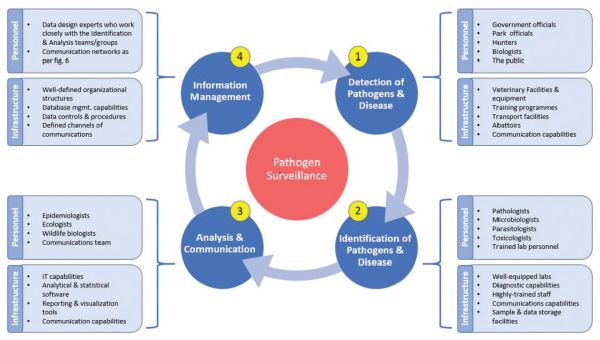
Figure 6: Four core components of an effective disease surveillance programme.
Both human health and animal health are inextricably dependant on the maintenance of functioning, healthy ecosystems meaning. There needs to be a shift away from the species-specific approach to disease towards the awareness that conservation of biodiversity and of healthy, functional ecosystems is vital to the health of individuals and populations, human and otherwise. Now, more than ever, we must expand our transdisciplinary collaborations and cooperation and truly operationalise One Health in a way we have thus far failed to do. One final point relates to the importance of continued surveillance for emerging diseases as the interface between wild animals, livestock and humans intensifies. Surveillance programmes need to implemented with specific goals so that the data generated can be used to document the health of wildlife and livestock populations, guide conservation efforts, categorise disease distribution, guide management strategies and trigger reactions in the case of novel diseases being detected in a new area (Figure 6). It is also essential that surveillance and management programmes include a continuous process of monitoring and re-evaluation so that the efficacy of management practices can be evaluated and adapted as needed.
Conclusion
In conclusion, the world has changed irrevocably as a result of Covid-19 and there is now no going back. It is time to finally press the ‘reset’ button and catalyse modes of stewardship for healthy ecosystems and a resilient natural world, which we are all completely dependent upon. Our community plays a central role in supporting decision-makers, politicians and organisations in understanding that intact and functional ecosystems provide the core infrastructure of health for all species on our planet and it is our duty as vets to listen, to translate science and to battle the current infodemic. Covid-19 is undeniably a symptom of ailing planetary health and it is now time to focus resources on the world stage and boldly engage in driving the paradigm shift necessary to ensure the biological integrity of the planet for current and future generations.
1. Emerging infectious diseases can be most accurately defined as:
a. Infections that have newly appeared in a population
b. Infections that have existed previously but are rapidly increasing in incidence
c. Infections that have newly appeared in a population or have existed previously but are rapidly increasing in incidence or geographic range
d. Infections that have existed previously but are rapidly increasing in geographic range
2. Approximately what percentage of human zoonotic diseases originate from wildlife?
a. 50%
b. 75%
c. 90%
3. Prevention of emerging infectious diseases in livestock populations needs to focus on:
a. Stringent biosecurity
b. Surveillance and control measures
c. Responsible use of veterinary medicines
d. All of the above
4. Which of the following definitions most accurately describes an ‘interface’?
a. The interaction of livestock and wildlife populations
b. The physical space in which some form of contact or shared use of resources occurs between wildlife, livestock and/or human populations
c. The interaction of wildlife and human populations
5. An ‘interface’ is often found:
a. At sites where humans have high contact rates with wild animals such as in wildlife markets
b. At forest buffer zones
c. In locations with substantial land-use change
d. All of the above
Answers: 1:C; 2:B; 3:D; 4:B; 5: D





